-
Publish Your Research/Review Articles in our High Quality Journal for just USD $99*+Taxes( *T&C Apply)
Offer Ends On
Anurag Modi, Pankaj Sharma*, Jitender Chaturvedi and Sanjeev Kishore
Corresponding Author: Pankaj Sharma Additional Professor Department of Radiodiagnosis All India Institute of Medical Sciences, Rishikesh, Uttarakhand, 249203 India.
Received: September 15, 2024 ; Revised: October 22, 2024 ; Accepted: October 25, 2024 ; Available Online: December 30, 2024
Citation: Modi A, Sharma P, Chaturvedi J & Kishore S. (2024) Comparison of Arterial Spin Labelling Perfusion Imaging Grade and Histopathological Vascular Density for Evaluation of Brain Tumors. J Can Sci Res Ther, 4(1): 1-8.
Copyrights: ©2024 Modi A, Sharma P, Chaturvedi J & Kishore S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Views & Citations
Likes & Shares
Background: Arterial Spin Labelling (ASL) is a novel MRI technique, that measures tissue perfusion (blood flow), by using magnetically labelled arterial blood water protons as an endogenous tracer. Our study was first prospective study from Himalayan belt of North India, wherein we tried to compare ASL perfusion imaging grade and histopathological vascular density for evaluation of brain tumors.
Aim and Objective: To compare arterial spin labelling perfusion imaging grade and histopathological vascular density for evaluation of brain tumors.
Material and Method: 40 patients who were referred to Department of Radiodiagnosis for evaluation of brain tumor, and who gave informed consent, were included in this prospective study, done over a period of 18 months. All patients underwent MRI, followed by surgical resection.
Result and Observation: When we compared performance of study parameters for predicting low versus high grade brain tumor, then we found that diagnostic accuracy was highest for mean CBF Ratio (Centre/Periphery) with cut off of 59.33, and area under ROC curve = 0.805.
Conclusion: High grade brain tumors displayed higher CBF (95.91 ml/min/100g) with use of ASL, as compared to low grade brain tumors (51.25 ml/min/100g). Cut off of 51.25 ml/min/100g had good diagnostic accuracy, with which we could distinguish between high grade and low-grade brain tumors, with sensitivity of 81.2% and specificity of 79%. ASL CBF grading also significantly correlated with histopathological grading of brain tumor.
Keywords: Arterial spin labelling, Grading, Histopathology, Vascular density, Brain tumor
INTRODUCTION
The most common primary benign brain tumor in adults is meningioma, while the most common primary malignant brain tumor is glioma. Malignant brain tumors are considered rare, as they merely account for 1% to 22% of all cancers in adults.
In the current practice of brain tumor management, treatment is adjusted according to staging; determined primarily based on histopathologic grading of the particular tumor. Hence, various studies are being conducted to determine, how various imaging modalities can predict the molecular subtype of a brain tumor, which may ultimately lead to survival benefit, due to ability to select most appropriate treatment strategy.
Magnetic Resonance Imaging (MRI) is imaging method of choice to localize, and to characterize brain tumors. Newer imaging techniques can provide functional and physiological information of brain tumors. Tumors derive their blood supply from the process of neoangiogenesis. Increase in neovascularity can be used as a surrogate marker for assessing tumor grade. Perfusion imaging is one such technique that measures blood flow to a brain region, or a tumor [1]. Many approaches exist to quantify brain perfusion such Positron Emission Tomography (PET), Dynamic Susceptibility Contrast Magnetic Resonance Imaging (DSC-MRI) and Computed Tomography Perfusion (CTP). However, these techniques require administration of intravascular contrast material.
Arterial Spin Labelling (ASL) is a novel MRI technique, that measures tissue perfusion (blood flow), by using magnetically labelled arterial blood water protons as an endogenous tracer [1-18]. ASL technique can estimate tumor neo-angiogenesis, which helps in tumors grading, guiding stereotactic biopsy, surgical planning & differentiating radiation necrosis from recurrent tumor. Our study was first prospective study from Himalayan belt of North India, wherein we tried to compare ASL perfusion imaging grade and histopathological vascular density for evaluation of brain tumors.
AIM AND OBJECTIVE
To compare arterial spin labelling perfusion imaging grade and histopathological vascular density for evaluation of brain tumors.
MATERIAL AND METHOD
40 patients who were referred to Department of Radiodiagnosis for evaluation of brain tumor, and who gave informed consent, were included in this prospective study, done over a period of 18 months. Patient with ongoing treatment for brain tumor, or patient with past history of treatment for brain tumor, or patient with history of recurrent brain tumor, or patient who failed to give informed consent were excluded from this study. All patients underwent MRI, followed by surgical resection.
All MRI scans were done on 3T MRI (GE Discovery 750 W, GE Healthcare USA) with dedicated brain coil.
Technique:
Following sequences were obtained
The images were analyzed using the workstation provided with the MRI scanner. For each tumor, region of interest (ROI) was drawn around both the enhancing and non-enhancing solid portions of the tumors, at all available axial levels of the ASL tumor blood flow map (Figure 1). ROIs were placed in the center, and in the periphery of the tumor. ASL perfusion of tumor was compared with the contralateral white matter cerebral blood flow (CBF). Areas of cyst, necrosis & gross hemorrhage were avoided. Regional cerebral blood flow (rCBF) value was calculated using the provided software.
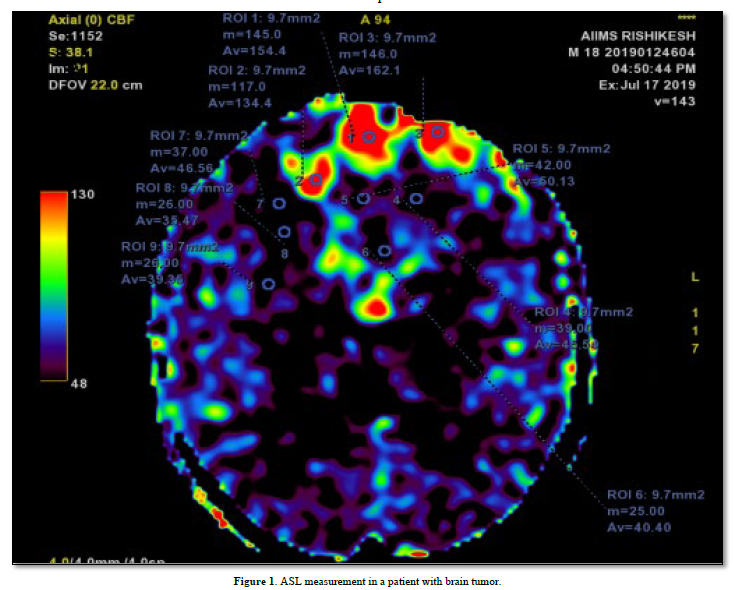
Histopathological vascular density grading was obtained after tumor was surgically removed, or biopsied. Endothelial cells were identified using CD 34 or Ki67 immunohistochemistry marker. Positively stained cells were identified under the microscope. % Area of positively stained cells were calculated per mm2 of section, for determining the histopathological grading.
OBSERVATION AND RESULT
40 patients were included in study population, with 24 patients having low grade brain tumor, and 16 patients having high grade brain tumor. The mean age (years) in low grade brain tumor was 36.71 ± 14.07, and 39.06 ± 17.73 in high grade brain tumor. There was no significant difference between the groups in terms of age in years (t = -0.446, p = 0.659).
In low grade tumors, 7 (29.2%) patients were in age group of 21-30 years, 6 (25.0%) patients were in age group of 31-40 years, 5 (20.8%) patients were in age group of 41-50 years, 3 (12.5%) patients were in age group of 51-60 years, 2 (8.3%) patients were in age group less than 20 years, and 1 (4.2%) patient was in age group of 61-70 years.
In high grade tumors, 5 (31.2%) patients were in age group of 41-50 years, 4 (25.0%) patients were in age group of 21-30 years, 3 (18.8%) patients were in age group less than 20 years, 2 (12.5%) patients were in age group of 71-80 years, 1 (6.2%) patient was in age group of 31-40 years, and 1 (6.2%) patient was in age group of 51-60 years.
There was male preponderance both in low grade (15 out of 24 patients), and high grade (12 out of 16 patients) brain tumor. Mean CBF Ratio (Centre/Periphery) was 47.53 ± 21.25 in low grade brain tumor, and 83.13 ± 33.34 in high grade brain tumor. Tumor grade ratio was 1.57 ± 0.53 in low grade brain tumor, and 3.11 ± 1.07 in high grade brain tumor.
There was 75% agreement in low grade brain tumors, and 100% agreement in high grade brain tumors, on comparison of ASL and histopathology report, when we graded brain tumor as Grade I, Grade II, Grade III and Grade IV, both on ASL and histopathology. However, there was 79.2% agreement in low grade brain tumors, and 100% agreement in high grade brain tumors, on comparison of ASL and histopathology report, when we graded brain tumor as low grade and high grade, both on ASL and histopathology.
In low grade brain tumor category, 10 (41.7%) patients had diffuse astrocytoma, 4 (16.7%) patients had meningioma, 3 (12.5%) patients had oligodendroglioma, 2 (8.3%) patients had pilocytic astrocytoma, 1 (4.2%) patient had central neurocytoma, 1 (4.2%) patient had ependymoma, 1 (4.2%) patient had ganglioglioma, 1 (4.2%) patient had hemangioblastoma, and 1 (4.2%) patient had pleomorphic xanthoastrocytoma.
In high grade brain tumor category, 8 (50.0%) patients had glioblastoma, 2 (12.5%) patients had medulloblastoma, 2 (12.5%) patients had pineal blastoma, 1 (6.2%) patient had anaplastic astrocytoma, 1 (6.2%) patient had anaplastic ependymoma, 1 (6.2%) patient had anaplastic meningioma, and 1 (6.2%) patient had atypical meningioma.
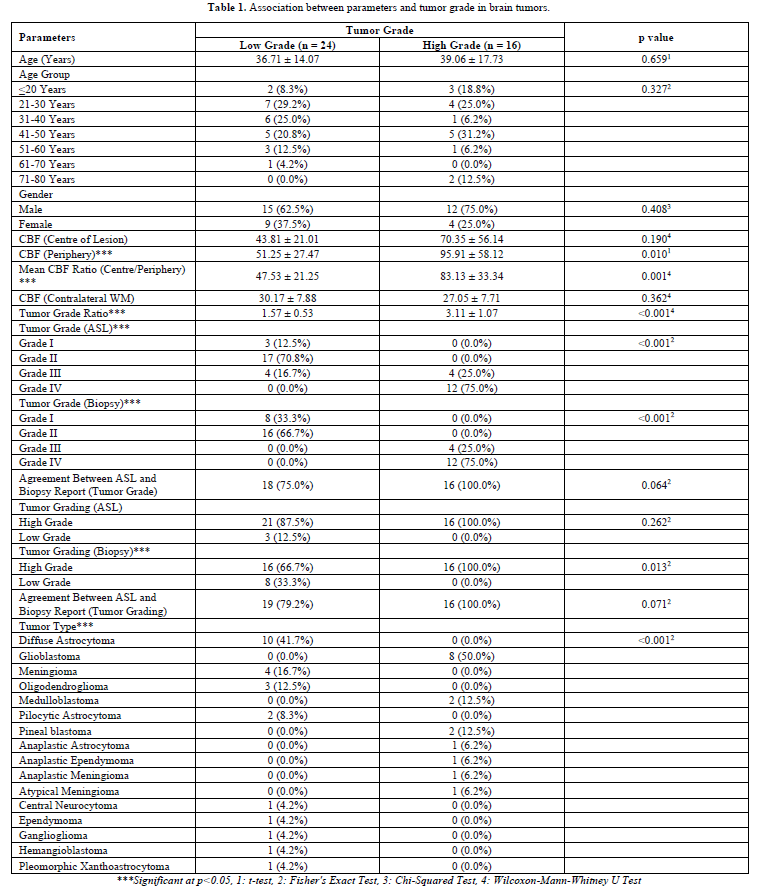
The following variables were significantly associated (p<0.05) with the variable 'Tumor Grade' (Table 1): CBF (Periphery); Mean CBF Ratio (Centre/Periphery); Tumor Grade Ratio; Tumor Grade (ASL): Grade I, Grade II, Grade III, Grade IV; Tumor Grade (Biopsy): Grade I, Grade II, Grade III, Grade IV; Tumor Grading (Biopsy): Low grade, High Grade; and Tumor Type.
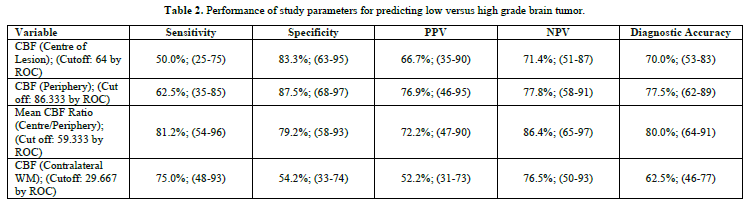
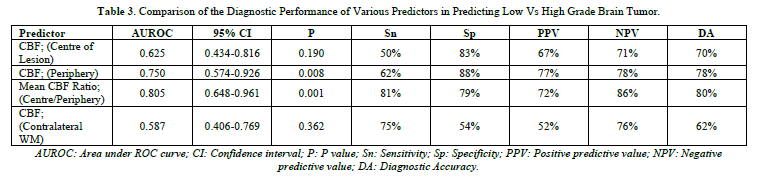
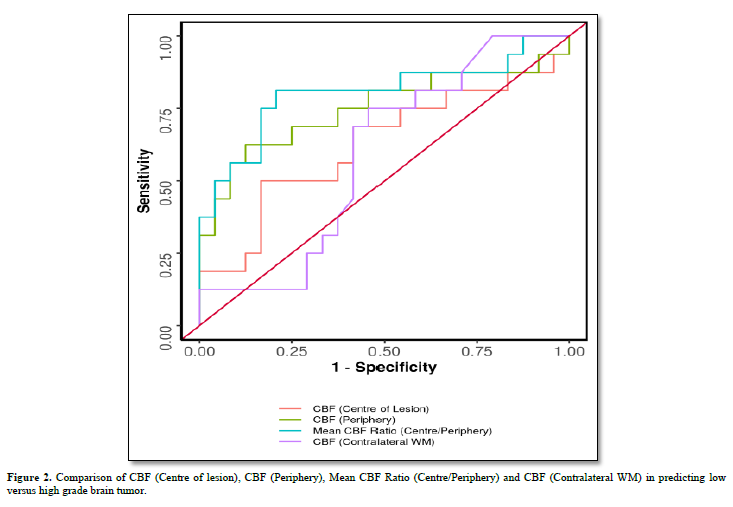
When we compared performance of study parameters for predicting low versus high grade brain tumor, then we found that diagnostic accuracy was highest for mean CBF Ratio (Centre/Periphery), with cut off of 59.333 (Table 2), and area under ROC curve = 0.805 (Table 3).
When we compared CBF (Centre of lesion), CBF (Periphery), Mean CBF Ratio (Centre/Periphery) and CBF (Contralateral WM) in predicting low versus high grade brain tumor; then we found that Mean CBF Ratio (Centre/Periphery), and CBF (Periphery) could significantly help in predicting low versus high grade brain tumor (Figure 2).
Trends based on our study:
DISCUSSION
ASL enabled us successfully to distinguish between low grade versus high grade brain tumors in different location, in a cohort of 40 patients. In this study, we drew 9 ROI in highly perfused regions of brain tumors; 3 ROI were placed in tumor periphery, 3 ROI were placed in center of tumor, and 3 ROI were placed in contralateral white matter. Mean tumoral CBF was calculated by taking the mean of tumor CBF at center and periphery of brain tumor. ASL tumor grade ratio was calculated by dividing the mean tumoral CBF with CBF in contralateral white matter. Tumoral grade ratio was finally compared with the histopathological grading. The algorithm we proposed yielded a simple classification with use of quantitative values, with an accuracy of 87.5%.
Tumor blood vessels have defective and leaky endothelium. Hypoxia or hypoglycemia that occurs in rapidly growing tumors, increases the expression of VEGF, which is not only a potent angiogenic factor, but also a potent permeability factor. VEGF leads to the development of neo-angiogenic vessels, which are immature and tortuous, and have increased permeability to macromolecules, due to large endothelial cell gaps, incomplete basement membrane, and absence of smooth muscles. These abnormal tumor vessels can be used as potential markers to assess the tumor grade. Measurement of tumor blood flow is a good surrogate marker for mean vascular density, a measure of angiogenesis and an important prognostic indicator in many human cancers.
Our study highlighted that high-grade brain tumors had greater CBF, as compared to CBF in low grade brain tumors. Average CBF was 95.91 mL/min/100 g in high grade brain tumor, while average CBF was 51.25 mL/min/100 g in low grade brain tumor. Meningioma was an exception in our study, as meningioma is a low-grade brain tumor; however, it showed CBF of 80.50 mL/min/100 g. Meningioma are hypervascular tumors, despite usually being benign in nature. Meningioma usually have large vessels, and vascular component sometime can exceed total tumor area.
In biopsy proven low grade brain tumors, 12.5% of the participants had grade I brain tumor on ASL grading; 70.8% had grade II brain tumor on ASL grading; 16.7% had grade III brain tumor on ASL grading; and 0.0% had grade IV brain tumor on ASL grading.
In biopsy proven high grade brain tumors, 0.0% of the participants had grade I brain tumor on ASL grading; 0.0% had grade II brain tumor on ASL grading; 25.0% had grade III brain tumor on ASL grading; and 75.0% had grade IV brain tumor on ASL grading.
In biopsy proven low grade brain tumors, 33.3% of the participants had grade I brain tumor on histopathology; 66.7% had grade II brain tumor on histopathology; 0.0% had grade III brain tumor on histopathology; and 0.0% had grade IV brain tumor on histopathology.
In biopsy proven high grade brain tumors, 0.0% of the participants had grade I brain tumor on histopathology; 0.0% had grade II brain tumor on histopathology; 25.0% had grade III brain tumor on histopathology; and 75.0% had grade IV brain tumor on histopathology.
The two methods agreed in 85.0% of the cases and disagreed in 15.0% of the cases. There was Near Perfect agreement between the two methods, and this agreement was statistically significant (Weighted Kappa = 0.833, p = <0.001). The disagreement observed between the two method was as follows: 2 (5.0%) cases classified as Grade I by Tumor Grade (Biopsy) were classified as Grade II by Tumor Grade (ASL); 3 (7.5%) cases classified as Grade I by Tumor Grade (Biopsy) were classified as Grade III by Tumor Grade (ASL); and 1 (2.5%) case classified as Grade II by Tumor Grade (Biopsy) was classified as Grade III by Tumor Grade (ASL).
37 (92.5%) of the participants had Tumor Grading (ASL): High Grade, and 3 (7.5%) of the participants had Tumor Grading (ASL): Low Grade. 32 (80.0%) of the participants had Tumor Grading (Biopsy): High Grade, and 8 (20.0%) of the participants had Tumor Grading (Biopsy): Low Grade.
Our results are in concordance with study done by Dangouloff-Ros V [14]. They described raised rCBF values in high grade tumor (more than 50 ml/min/100 g), as compared to low grade tumor (less than 50 ml/min/100 g). In our study, we noted mean tumoral CBF as 95.91 ml/min/100g in high grade tumor, and 51.25 ml/min/100g in low-grade tumor.
Noguchi T [12], also calculated ASL brain tumor grading, and compared it with histopathological microvascular density. They measured % signal intensity, mean tumoral CBF and % vessel in 35 patients. Positive correlation was noted between % signal intensity, mean tumoral CBF and % vessel. Due to small sample size, correlations in meningioma, schwannoma and hemangioblastoma didn’t reached any statistical significance. In our study, meningioma didn’t show positive agreement with histopathological grading; due to its hypervascular nature. Also, hemangioblastoma didn’t reached any statistical significance, due to small sample size. We didn’t have any schwannoma in our sample size. We only measured tumoral CBF as a quantitative measurement (not % signal intensity), because of magnetic susceptibility artefacts, and due to low signal-to-noise ratio.
Ma [16], measured ASL-rCBF, DSC-rCBV and DSC-rCBF in diagnosing glioma grade, prior to surgery. However, we measured only ASL-rCBF (as rCBV can only be calculated on DSC). ASL-CBF for low grade glioma was 50.64 ml/100g/min, and for high grade glioma was 88.03ml/100g/min. In our study, ASL-CBF for low grade tumor was 51.25 ml/100g/min, and for high grade tumor was 95.91 ml/100g/min. Moreover, we included both gliomatous and non-gliomatous tumors in our study. Ma [16], also calculated area under ROC curve for high grade glioma, found difference in area under ROC curve when they evaluated ASL-rCBF, DSC-rCBV and DSC-rCBF. ASL-rCBF had largest area under ROC curve (0.836). In our study, area under ROC curve for high grade tumors was 0.805. So, our results are in concordance with study done by Ma [16].
Razak [11], used different methods of ASL for grading low grade versus high grade glioma. They also tried to differentiate lymphoma from glioma, and cystic glioma from abscess. However, we did not cover lymphoma (due to lack of WHO grading for lymphoma). We didn’t include infective lesions in our study. Razak [11], also calculated area under ROC curve; and this value was found to be 0.813 for grade II versus III brain tumors; 0.964 for grade II versus IV brain tumors; and 0.872 for grade III versus IV brain tumor. In our study, area under ROC curve was calculated as 0.805 for high grade versus low grade brain tumor.
Our study had several limitations. 5 patients in our study were excluded because of bleeding in brain tumor. This bleeding prevented the use of our algorithm in these lesions, because of the technical artefact and vascular artefacts, which are due to delayed arterial transit time. Few patients were also eliminated from our study, due to movement artefacts, and due to magnetic susceptibility artefacts. Physiological hypoperfusion/hyperfusion also imposed challenge, as normal brain parenchyma tissue also mimicks tumoral tissue.
ASL has few disadvantages:
However, ASL has several useful advantages:
We arbitrarily selected the most perfused/vascularized area of the neoplastic tissue. Non-enhancing necrotic areas, hemorrhagic and cystic areas were not selected for measuring ASL in our study. ASL calculation method was same for all brain tumors (like same ROI volume, same TR and TE), thereby preventing any bias; since the most vascularized area only was selected for tumors with low CBF. There was limited correlation between CBF and microvascular density, because of our inability to perfectly match pathology sample with region of interest on CBF map (except in cases of small biopsy). Our study was mainly performed with 3T MRI. This is in line with recent recommendations for ASL, which advise use of 3T MRI.
In conclusion, ASL is an accurate tool for classification of brain tumors on MRI based on CBF, as high-grade brain tumors display higher CBF, as compared to low grade brain tumors.
CONCLUSION
High grade brain tumors displayed higher CBF (95.91 ml/min/100g) with use of ASL, as compared to low grade brain tumors (51.25 ml/min/100g). Cut off of 51.25 ml/min/100g yielded good accuracy, with which we could distinguish between high grade and low-grade brain tumors, with sensitivity of 81.2% and specificity of 79%. ASL CBF grading also significantly correlated with histopathological grading of brain tumor.
Possible Direction for Future Research
Individualized treatment of brain tumor patients, based on principles of precision medicine, including individual patient profile, gene sequencing, with use of dedicated MRI coils and advanced MRI software, which will result in higher resolution to detect perfusion in small deep brain structures; which will ultimately expand the clinical utility of ASL.
No Files Found
Share Your Publication :