-
Publish Your Research/Review Articles in our High Quality Journal for just USD $99*+Taxes( *T&C Apply)
Offer Ends On
Andrey Manov*, Ashan Hatharasinghe and Sadik Acaralp
Corresponding Author: Andrey Manov, MD, Associate Program Director of Internal Medicine, Sunrise health Consortium GME Program, Las Vegas, NV and Professor, University of Nevada, Reno Medical School, NV. 5292 Painted Sunrise Dr., Las Vegas, NV, 89
Received: October 11, 2020 ; Revised: November 05, 2020 ; Accepted: November 09, 2020
Citation: Manov A, Hatharasinghe A & Acaralp S. (2021) Effective Treatment of Secondary Hypogonadism with Clomiphene Citrate in a Male Patient with Secondary Polycythemia and History of Transient Ischemic Attack. Ann Clin Case Stud Rep, 1 (1): 1-4.
Copyrights: ©2021 Manov A, Hatharasinghe A & Acaralp S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Views & Citations
Likes & Shares
In this case report we discuss a patient with secondary hypogonadism, who was placed on chronic testosterone replacement therapy despite having a history of transient ischemic attack (TIA). The patient developed secondary polycythemia with a hematocrit of 54.2%, therefore requiring discontinuation of testosterone intramuscular therapy. We discuss the implications of testosterone replacement therapy and how we achieved successful treatment of secondary hypogonadism with an off-label treatment - clomiphene citrate discovered.
Keywords: Transient Ischemic Attack, Testosterone Replacement Therapy, Hypogonadism
INTRODUCTION
Case Description
A 44-year-old male with testosterone deficiency diagnosed 3 years ago by his primary care provider with an abnormally low serum AM total testosterone level (125 ng/dl), low free testosterone level (40 pg/ml) and normal LH, FSH, presented to establish care in our primary care clinic. He was currently taking testosterone cypionate 200 mg injections every two weeks and had been on testosterone for the past 3 years. He notably was diagnosed with a transient ischemic attack at age 38 (3 years prior to testosterone replacement therapy being started) after being hospitalized for left upper extremity weakness and facial numbness. Brain imaging including CT of the brain without contrast, MRI of the brain, and MRA of the brain were negative at that time.
Despite history of TIA, the patient was placed on testosterone therapy 3 years later and was unable to be weaned off it due to worsening of symptoms of hypogonadism. A repeat MRI of the brain prior to starting testosterone was also negative for pituitary tumor, and no other causes such as hyperprolactinemia, primary hypothyroidism, hemochromatosis, or other pituitary hormonal deficiencies where Testosterone replacement therapy is the mainstay treatment for both primary and secondary hypogonadism [1,2]. Clinical benefits of testosterone include improvement in muscle mass, libido, and bone density. However, there are potential risks associated with it, including hypercoagulability, prostate cancer, worsening sleep apnea, cardiovascular disease etc. There are contraindications for treatment as per current guidelines from the American Association of Clinical Endocrinologists (AACE) [1].
Therefore, initiating testosterone should be done with caution and after excluding breast cancer, prostate cancer. Additional exclusion parameters include PSA above 4 ng/ml or above 3 ng/ml in high-risk patients such as African Americans or patients who have a first degree relative with prostatic cancer, untreated congestive heart failure, untreated severe sleep apnea, thrombophilia, stroke, transient ischemic attack (TIA) or myocardial infarction within 6 months, hematocrit above 50%, or if the patient seeks fertility [1].
Given the high prevalence of cardiovascular disease worldwide, testosterone replacement therapy may not be a viable option for every patient.
Another potential medical treatment for hypogonadism in male patients, especially those desiring fertility and not having primary hypogonadism, is clomiphene citrate selective estrogen receptor modulator (SERM) or less frequently used
letrozole/anastrozole- aromatase inhibitors [3-6]. Although they are not FDA approved for treatment of hypogonadism, several retrospective studies and randomized trials have demonstrated improvement in serum testosterone levels with clomiphene and aromatase inhibitors [4-6].
Other potential medical treatment for patients desiring fertility is with the use of hCG intramuscular injections [6].
Other potential interventions that should be considered to increase testosterone levels include- adequate treatment of diabetes mellitus, obesity, obstructive sleep apnea, dyslipidemia and stress reduction and/or depression due to biofeedback mechanisms effecting testosterone levels. If varicocele is present it should be treated with surgical repair.
In addition to TIA, the patient’s history included class 2 obesity (BMI of 37 kg/m2), depression, and metabolic syndrome. Given his metabolic risk and history of TIA, the long-term testosterone therapy raised concerns for an increased risk of major adverse cardiac events, stroke, worsening hematocrit, or rising PSA levels.
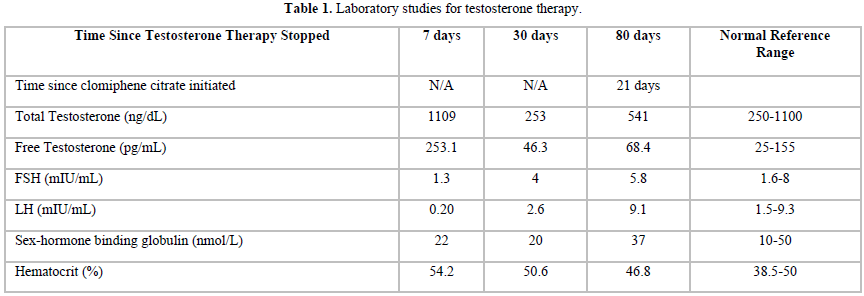
The laboratory parameters were not followed in the past by his previous provider who was treating the patient with testosterone injections. Laboratory studies were ordered by our team and drawn approximately seven days after the last testosterone injection. Total testosterone level was elevated (1109 ng/dL) along with a suppressed FSH of 1.3 mIU/mL, and a suppressed LH of 0.2 mIU/mI (Table 1). A hematocrit of 54.2 % confirmed the diagnosis of polycythemia likely secondary to testosterone replacement therapy. The patient was also referred for a sleep study due to high-risk stratification for obstructive sleep apnea (OSA) and complaint of new onset insomnia. Sleep study results confirmed severe OSA and he was started on CPAP therapy. Obesity was managed with diet which included a 1500 calories/day restriction, walking 45 min per day, and phentermine 15 mg. His depression was managed with doxepin which he was taking previously.
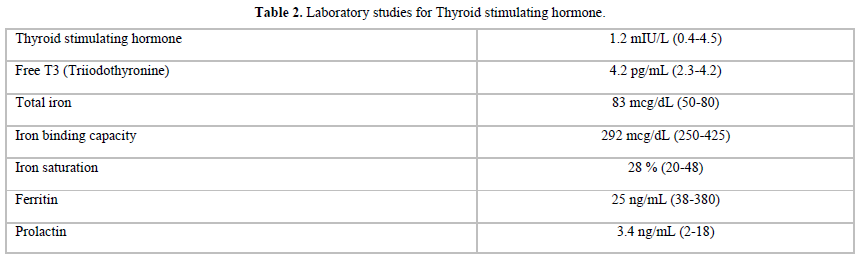
The testosterone intramuscular injections were discontinued due to the contraindications regarding his hematocrit above 54% [3]. There was also concern for the high risk of a repeat cardiovascular event in a patient who had now polycythemia. Laboratory studies were repeated (Table 1) after the patient had been off testosterone replacement therapy for approximately 30 days. The patient’s TSH, free T4, and prolactin were normal. An iron panel was also somewhat unremarkable with a transferrin saturation of 28% and ferritin level at the low-normal level of 25ng/ml. The hematocrit improved to 50.6 % but was still slightly elevated. Chromosomal analysis was also performed and showed normal 46 XY karyotype with normal 6-band patterns.
Based on these results, along with normal testicular size on testicular ultrasound, and normal LH and FSH with significantly low testosterone levels 3 years ago, the patient’s condition was determined to be consistent with secondary idiopathic hypogonadism.
The patient followed up 7 weeks after stopping the testosterone injections, yet despite the treatment of OSA, improvement in obesity (weight decreased from 251 lbs to 237 lbs), and successful treatment of his depression with doxepin, the patient still complained of loss of libido, no morning erections, and inability to have sexual intercourse.
Due to no improvement in clinical status with lifestyle improvements and a decreasing AM testosterone level from 283 ng/dl to 253 ng/dl and free testosterone decreasing to 49 pg/ml, the decision was made to start the patient on clomiphene citrate 25 mg daily.
Repeat laboratory testing after the patient had been on clomiphene citrate for four weeks, and off of testosterone replacement therapy for approximately 80 days showed significant improvement in testosterone levels, FSH, and LH (Table 1). Hematocrit had also normalized to 46.8 % indicating resolution of polycythemia. The patient reported satisfactory outcomes with normalization of his libido, morning erections and ability to have sexual intercourse.
DISCUSSION
We have presented a patient with hypogonadism who was treated with testosterone injections. The hypogonadism was central and there were some physiological components contributing to it- obesity, OSA, depression. The testosterone injections were stopped due to a hematocrit of 54.2%, and we attempted to treat the hypogonadism with lifestyle intervention, yet despite favorable outcomes with obesity, OSA, and depression, the testosterone decreased to low normal ranges, and this was compounded by loss of libido, morning erections and ability to have sexual intercourse which necessitated treatment with an alternative drug.
The etiology of the central hypogonadism was most likely late idiopathic. MRI done 3 years ago when the testosterone was started was negative. There were several physiological factors contributing to the disease that were addressed and treated, but the central hypogonadism persisted and caused these symptoms caused significant distress to the patient which was the primary reason to seek alternative treatment.
Clomiphene citrate is a selective estrogen receptor modulator. In the context of male hypogonadism, it works by inhibiting negative feedback to promote gonadotropin -releasing hormone, LH, and FSH hormone release. As we saw with our patient his LH, FSH, total testosterone, and free testosterone significantly improved with clomiphene citrate. Given its mechanism of action, clomiphene cannot be used in the treatment of primary hypogonadism. Of note, it is also frequently used to increase fertility in men (by increasing the spermatogenesis) however this was not the goal for treatment in our patient.
In our case, the patient’s libido significantly improved with clomiphene. Ramasamy [7] reported that clomiphene achieved testosterone levels comparable to testosterone gels. They also noted libido improved similarly with testosterone gel. However, the improvement of the testosterone level and libido were less than those observed with testosterone intramuscular injections [7].
Most importantly, the polycythemia resolved in our patient with clomiphene citrate compared to an increase with testosterone injections. The patient’s testosterone level also improved to a normal level, morning erections returned, and he was able to have sexual intercourse. The prevalence of polycythemia in men treated with clomiphene for hypogonadism was markedly lower than that in men on testosterone therapy [8] and there is no significant risk of polycythemia in men treated with clomiphene citrate compared to those treated with testosterone for hypogonadism [8,9].
There is a limited amount of research regarding long term clomiphene use given its off-label use. Katz [10] in a prospective study over 19- months reported safety and efficacy in treating hypogonadism in young men. Chandrapal [9] found that the drug significantly increased testosterone levels without increasing the prostatic specific antigen or hematocrit.
In summary, we think clomiphene citrate is a cost- effective treatment of secondary hypogonadism once a pituitary tumor, primary hypothyroidism, prolactinoma, hemochromatosis or primary hypogonadism are ruled out. It is a viable option to treat men who develop secondary polycythemia while on testosterone replacement therapy especially if there are any other cardiovascular risks.
No Files Found
Share Your Publication :