-
Publish Your Research/Review Articles in our High Quality Journal for just USD $99*+Taxes( *T&C Apply)
Offer Ends On
Sonia Sangwan*
Corresponding Author: Sonia Sangwan, PhD, Ex Scholar Department of Dairy Chemistry, NDRI, Karnal, India
Received: December 16, 2021 ; Revised: December 27, 2021 ; Accepted: January 20, 2022
Citation: Sangwan S. (2022) Nanotechnology and Dermatology. J Derm Allerg Res, 1(1): 1-6.
Copyrights: ©2022 Sangwan S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Views & Citations
Likes & Shares
Abstract
Nanotechnology at the atomic scale of 100 nm or less is regarded as the most coming up technology of the 21st century. It is considered a big boon in dermatology and its application in electronics, chemicals, environmental protection, and biological medicine. Nanospheres have substituted the usage of the conventional delivery system. These days N.P.s are used in multiple cosmeceuticals as creams and lotions. They are used for skin, hair, nail, and lip care for conditions like wrinkles, photoaging, hyperpigmentation, dandruff, and hair damage have widespread use. These novel nanocarriers have enhanced skin penetration, controlled and constant drug release, higher stability, site-specific targeting, and high entrapment efficiency. However, these nanosized particles are likely to increase an unnecessary endless toxicological effect on animals and the environment, although their toxicological effects associated with human exposure are still unknown. These N.P.s can enter the host systems via skin spores, debilitated tissues, injection, olfactory, respiratory and intestinal tracts. These uptake routes of N.P.s may be intentional or unintentional. Their entry may lead to various unfavorable biological effects. There are possibilities for nanoparticles to penetrate through the skin and cause health hazards. This review on nanotechnology used in dermatology highlights the positive and negative aspects, toxicity, and cosmetics regulations.
INTRODUCTION
The new scientific advancement of engineering nanoparticles (N.P.s) at the atomic scale (diameter<100nm) has led to many new and useful wide uses in chemical, medical imaging, disease diagnoses, drug delivery, cancer treatment, gene therapy, etc. Recently N.P.s have been used in various skin creams and sunscreen lotions. During the era of 4000BC, nanotechnology was used by the Egyptians, Greek, and Romans, with the concept of hair dye preparation utilizing nanotechnology [1]. Egyptians started using N.P.s in around 4000BC, and later, Greeks, Romans, Chinese, Japanese, and Americans started using N.P.s for cosmetics creams and lotion. Before the 20th century, women used cosmetics made up of household kitchen items like turmeric (Haldi), curd, honey and aloe vera secretly. By the 20th century, use of the cosmetics has become an open fashion [2,3].
The cosmetic products (Cosmeceuticals) incorporate biologically active ingredients having therapeutic benefits on the skin surface when applied. These are used as a facial application to enhance looks and beautify. Cosmeceutical products have significant restorative and cosmetic efficacy on the skin. They are useful for hair damage, wrinkles, photoaging, skin dryness, dark spots, uneven complexion, hyperpigmentation, and so on [4].
These nanosized particles are likely to boost needless infinite toxicological effects on animals and the environment, although their toxicological effects linked with human exposure are still unidentified. These ultrafine particles can enter the body through skin pores, debilitated tissues, injection, olfactory, respiratory and intestinal tracts. These uptake routes of N.P.s may be intentional or unintentional. Their entry may lead to various diversified unpleasant biological effects.
Despite the enormous benefits of nanoparticles, little is known about the short-term and long-term health effects. Due to the toxicity of N.P.s, safety concerns have been raised. The present article reviews N.P.s positive and negative aspects.
ADVANTAGES AND POSSIBLE SIDE EFFECTS OF N.P.S ON SKIN
There are several uses of Nanocosmeceuticals as they offer the controlled release of active substances due to physical or chemical interaction among the components, drug composition and the method of preparation. They are used in hair care pre, such as treating hair loss and preventing hair from turning grey in the form of shampoos. Nanocosmeceuticals give prolonged fragrances that last longer. N.P.s make the skincare formulations more effective and increase the efficacy of sunscreens by improving U.V. protection in them. The N.P.s have surface area due to small size, which permits the active transfer and penetration of the active constituents into the skin, thus enhance skin hydration. Nanocosmeceuticals have high entrapment efficiency and good sensory properties and are more stable than usual cosmetics. Most nanoparticles are appropriate for both lipophilic and hydrophilic drug delivery. Nanomaterials are widely used to prepare antiwrinkle creams, moisturizing creams, skin whitening creams, hair repairing shampoos, conditioners, and hair serums [5,6]. Several positive natures of Nanocosmeceuticals are discussed in Figure 1.

Cosmeceuticals for skincare products improve the skin texture and functioning by stimulating collagen growth by combating the damaging effect of free radicals. They maintain the skin healthier by keeping the structure of keratin in fine condition. In sunscreen lotions, zinc oxide and titanium dioxide nanoparticles are the most effective minerals which protect the skin by penetrating the deep layers of skin and make the product less greasy, less smelly, and transparent [7,8]. The antiaging nanocosmeceutical products incorporate nanocapsules, liposomes, and nanospheres with evident benefits such as collagen renewal, skin transformation, and firming and lifting the skin [9].
Hair nanocosmeceutical products comprise shampoos, conditioning agents, hair growth stimulants, coloring, and styling products. The hair follicle, shaft targeting, and increased quantity of active ingredients are attained by built-in properties and a certain size of nanoparticles. Nanoparticles present in shampoos form protective film over scalp hair follicles and thus preserve moisture within the cuticles of scalp [10]. Conditioning nanocosmeceuticals agents impart softness, shine, silkiness, and gloss and improve disentangling of hair. Novel carriers of NPS have the property of making hairs shiny, glossy, repair damaged cuticles and restoring their texture.[11].
Lipstick and lip balm are used for lip care. A variety of nanoparticles can be merged into lip gloss and lipstick to soften the lips by hindering trans epidermal water loss [12] and thus preventing the pigments from migrating from the lips and maintaining color for a longer period. Lip care products hydrates and outlines the lips, and fills wrinkles in the lip contour [13].
The nail care products having N.P.s have superiority over conventional products. Nanotechnology nail paints have improved toughness, fast dryness, durability, chip resistance, and ease of application due to elasticity [14]. New approaches such as combining silver and metal oxide nanoparticles have antifungal properties in nail paints to treat toenails due to fungal infections [15].
TOXICITY OF NANOCOSMECEUTICALS
Size is the main factor in determining the potential toxicity of a particle [16]. Other properties of nanomaterials that influence the toxicity of cosmeceuticals, chemical composition, shape, surface structure, surface charge, aggregation and solubility [17], and the presence or absence of functional groups of other chemicals. Other reasons for the drawbacks associated with skin creams are producing many oxygen species, oxidation stress, inflammation, damage to DNA, proteins, and membranes caused by nanoparticles. The regulatory agencies imposed no strict scrutiny to approve and regulate N.P.s skin creams and lotions (Nanocosmeceuticals). No clinical trials are required to support nanocosmeceuticals, thus raising a concern of toxicity after use [18,19].
The scientific committee on Consumer Products (SCCP) has raised concerns about the toxicity of nanoparticles due to overuse in cosmetics that are used topically over the skin. The royal society world's oldest scientific organization, is worried whether NPs can enter the bloodstream, be taken up by cells and show their effect [21]. This has raised issues regarding chronic effects due to long term use all over the globe [22].
Several workforce and customers exposed to nanoparticles are rising because of the growing production and wide application of cosmeceuticals products that contain nanomaterials. Apprehension has been raised on the possible dangers of nanomaterials' skin penetration after their application to the skin [23]. The toxicity of nanoparticles depends on various factors like surface properties, coating, structure, size, and ability to aggregate. These factors can be modified and maneuvered in the production process. Nanoparticles due to poor solubility may cause cancer and can show more specific toxicity [24]. Health hazards may occur due to the surface area of nanoparticles when compared with the same mass concentration of large particles. Toxicity can also result due to the chemical composition of nanoparticles which is absorbed by the skin. The particle size of NPs decides the toxicity: the smaller the size of the nanoparticles, the greater the surface area to volume ratio, resulting in higher chemical and biological reactivity.
The degree of exposure and the route of entry of N.P.s decide toxicity. Inhalation, ingestion, dermal, portal, intravenous, mucous membrane, auditory and ocular routes are the possible routes humans can get exposure to the nanoparticles [25].
Various routes of exposure (Figure 2)
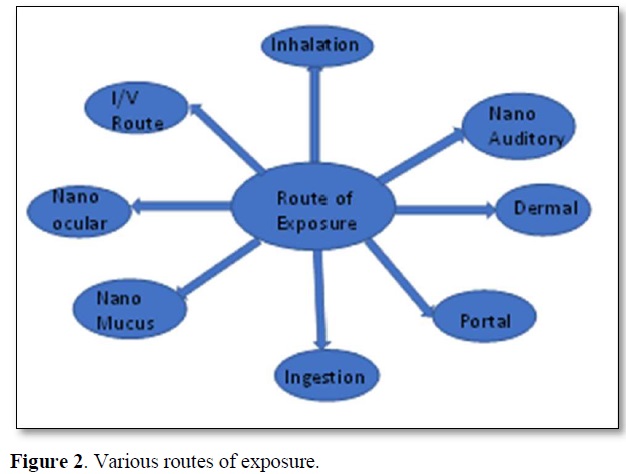
Inhalation
The most common way for exposure to airborne nanoparticles is inhalation. Consumers may inhale the nanoparticles and get exposure through the respiratory while consuming the products, such as perfumes and powders. Aerosol and workers can get vulnerable to the nanoparticles during production. Evidence from the studies conducted on animals suggests that most nanoparticles inhaled enter the pulmonary tract. Some may go up via nasal nerves to the brain and gain access to other organs via blood [26]. Experiments on carbon nanotubes have shown that chronic exposure, interstitial inflammation and epithelioid granulomatous lesions are caused in the lungs. Some carbon-based fullerenes might oxidize cells or may be hazardous when inhaled [27]. Ultrafine particles resulted in more lung injury. When exposed to the intratracheal route, gold nanoparticles of sizes 2, 40, and 100 nm were located in the liver and macrophages. It has been proved that the exposure to TiO2 of particle size 20 nm even at low doses destroys DNA, whereas 500 nm TiO2 have a small ability for DNA strand breakage [28].
Ingestion
Nanomaterials may be gulped in the body from unintentional transfer from hand to mouth. Nanoparticles can be consumed from cosmeceuticals that are applied on lips or mouth like lipsticks, lip balms, lip gloss, and so on [29]. According to the studies, nanomaterials rapidly pass out of the body after ingestion, but sometimes some may be taken up to travel to the organs. Studies on the layers of pig skin show that certain nanomaterials can penetrate the layers of the skin within 24 h of contact with skin [30]. When mice were orally fed with zinc oxide nanoparticles at 20 nm and 120 nm at different doses, the spleen, heart, liver, bones, and pancreas became the target organs. Copper nanoparticles are found in a variety of commercially available cosmeceuticals. Mice exhibited toxicological effects and serious injuries to internal organs when exposed to copper nanoparticles [31]. Silver nanoparticles are widely utilized in antimicrobial formulations for wound dressing and now are being used in body soaps, facial creams, lotion and toothpaste. The lethal silver concentration for bacteria is the same lethal concentration for both fibroblasts and keratinocytes [32]. Various studies on rats suggest that silver nanoparticles, when exposed to rat neuronal cells, led to size decrease and irregularities in shape and demonstrated that mouse germline stem cells, even at low concentrations of silver nanoparticles, reduced the function of mitochondria and cell viability drastically. When mice were fed gold nanoparticles of 13.5 nm orally, a significant decrease in the RBCs, body weight, and spleen index were observed [33].
Dermal Routes
The derma (epidermis & dermis) is the body’s largest organ, which is the superficial covering that protects the underlying internal organs against external exposure. Its other main functions are sensation, temperature regulation, vitamin D synthesis, and vitamin B folates protection.
The skin barrier alterations - such as wounds, scrapes, or dermatitis conditions could affect nanoparticle pierce the skin thus is a possible route of exposure and should be kept in mind. Debilitated skin is a good route for the entry of finer and even larger particles (0.5-7 µm) [34].
Intracellular, transcellular, and trans follicular are the three routes by which penetration occurs through the skin [35]. Fullerenes are recently being used in cosmetics like moisturizers and face creams, but their toxicity is not fully understood. The face creams that have fullerenes incorporated are found to cause damage in the fish’s brain and have toxic effects on the liver cells in humans [36]. Some studies demonstrated that fullerene-based peptides could penetrate intact skin, and their traversal into the dermis could be easy due to mechanical stressors. The various studies have shown that engineered nanoparticles like single or multiwall carbon nanotubes, quantum dots with surface coating, and nanoscale titania can cause changes in gene or protein expression and have harmful effects on epidermal keratinocytes and fibroblasts [37]. The nanoparticles of titanium dioxide and zinc oxide present in sunscreens may affect health as Titanium dioxide and zinc oxide produce ROS and free radicals when exposed to ultraviolet (U.V.) radiations, which have the potential for inflammation and oxidative stress and can destroy membranes, proteins, RNA, DNA, and fats within cells [38]. Research on TiO2 nanoparticles toxicity has shown that when these nanoparticles were subcutaneously injected in to the pregnant mice, they were carried by the offspring. There was a decreased sperm formation in male offspring. Nanoparticles of cobalt-chromium have the capacity to cross the skin barrier and damage fibroblast in humans [39].
During occupational exposure, NPs can enter in body even through intact skin human and pigs and this depends upon various factors like size, shape, water solubility, and surface coating of NPS. The topical application of raw SWCNT to nude mice has been shown to cause dermal irritation. In vitro studies using primary or cultured human skin cells have demonstrated that carbon nanotubes can enter cells and reduce cell viability by setting free pro-inflammatory cytokines, oxidative stress [40,41]. In addition, nanoparticles may enter the body through wounds, with particles migrating into the blood and lymph nodes [42].
Penetration of NPs is minimum in the tough uppermost stratum corneum of the skin and so is toxicity. There is slight irritation only. Any perturbations to the skin, such as an open wound, cut, or alteration to this skin barrier, could expose N.P.s to viable skin cells [43,44]. The skin is Orifices of hair follicles and glands on the skin surface provide alternative entrance routes to small size NPs present in cosmetics creams and lotions.
CONCLUSION
Recently, nanotechnology is used widely in dermatology, cosmetics, and biomedical applications due to its beneficial effect. Cosmetics are manufactured by using new and different delivery systems with help of nanotechnology. These new delivery systems have high potential in achieving controlled and targeted delivery, site-specificity with better stability and prolonged action. There are high controversies regarding the toxicity and safety of nanomaterials in cosmetics. Careful research is required to study the safety profile of the nanomaterials regarding health hazards and toxicity. Nanoproducts should be manufactured in such a way that there are minimum health hazards to the customers. As clinical trials are not necessary for approval of cosmetics, the manufacturing companies do not carry out clinical trials to save money.
The combination of hazard and production should go hand in hand to reduce potential acquisition of N.P.s through the International Standards Organization (ISO), good manufacturing practices (GMP), and good laboratory practices (GLP). Suitable quality control procedures should be part of the process to ensure N.P.s product safety and quality of the company quality assurance scheme. Also, the manufacturing industries of nanotechnology should work hand in hand with the health and hazard risk assessment to establish a lower health risk of any type emanating from the production and use of N.P.s. Lastly, stringent laws should be imposed on the regulation and safety of cosmeceuticals and nanoparticles used in them.
REFERENCES
No Files Found
Share Your Publication :