-
Publish Your Research/Review Articles in our High Quality Journal for just USD $99*+Taxes( *T&C Apply)
Offer Ends On
Otar Shainidze*, Nodar Beridze and Shota Lamparadze
Corresponding Author: Otar Shainidze, Batumi State University “Shota Rustaveli”, Agroecology and Plant Protection Department, Faculty of Technology, Batumi, Georgia.
Received: September 19, 2023 ; Revised: October 22, 2023 ; Accepted: October 25, 2023 ; Available Online: November 17, 2023
Citation: Shainidze O, Beridze N & Lamparadze S. (2023) The Main Pathogen of Radish (Raphanus Sativus L.) and Their Biocontrol in Adjara, Georgia. J Agric For Meterol Stud, 2(2): 1-7.
Copyrights: ©2023 Shainidze O, Beridze N & Lamparadze S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Views & Citations
Likes & Shares
The antifungal activity of 18 different plant species known for their medicinal value was investigated. Medicinal plants and pathogenic fungi were collected in different geographical zones of Adjara, Georgia. Plant material were cut into small pieces and dried at room temperature for one week. Fungal species identification was based on the morphological characteristics of single spored isolates. 17 species of fungi have been identified. 7 species of fungi involved in forming of consortium on root vegetable of Radish plants have been specified as well. Long-term monitoring has shown us that the mycobionts found on Radish, the most dangerous and harmful 4 pathogen of which the dominant species is Hyaloperonospora brassicae F. sp. raphani. Among the 21 extracts evaluated, 5 extract shoved mycelia inhibition 80.75-98.33%, 3shoved moderate effect of 75.56 - 79.32% and 6 showed least 31,4 % - 57.27%. The remaining 7 extracts from leaf of Sambucus ebulus, Ricinus communis, Betonica officinalis, Jasminum officinal, Pimpinella anisum, Pyrethrum vulgare and Althae officinalis showed no antifungal activity against the H. brassicae F. sp. Raphani. It was established that Rubia coradifolia, Inula racemosa, Stevia rebaudiana and Panax ginseng extracts can be emerged as safe alternatives to replace chemical fungicides against pathogens.
Keywords: Hyaloperonospora brassicae F. sp. raphani, Leaf extract, Antifungal activity, Inhibition effect, Biological control
INTRODUCTION
Radish (Raphanus sativus L.) is a vegetable cultivated mostly in the temperate areas for the root, which is succulent. It is very popular in Adjara (Georgia), mostly in the cold areas, due to its storage capacity. More recently, radish and other cruciferous vegetables (members of the Brassicaceae) have been recognized as important sources of chemoprotective phytochemicals in the diet. Radish is a productive vegetable based on biomass per area of cultivation. However, this crop is affected by many diseases, particularly those caused by fungi. Among fungi Radish downy mildew is an is an ecological and economically important disease in main production areas Georgia wide. We can find quite a lot of material about radish downy mildew is found in the works of various authors [1-7]. The orography of the Adjara and the edaphic-climatic conditions (Conditions conducive to the development and spread of pathogens) supports the widespread and development of Radish downy mildew causing agents.
Often, widespread diseases in the study area almost completely destroy the radish crop [8-10]. It is increasingly becoming a limiting factor for the successful cultivation of Radish in these regions. Several fungicides recommended for controlling these pathogens not only add up to human and environmental hazards but also be inconsistent and insufficient to deal with this disease. However, the application of this agrochemical is increasingly restricted due to the harmful effects of pesticides on biotic and abiotic factors [11]. In many countries, the use of fungicides has been banned due to their polluting nature to have safe methods for plant disease control in sustainable agriculture there is a need for reducing the use of synthetics chemicals fungicides thereby replacing them with biocides with plant origin. The medical plants are rich sources of numerous bioactive secondary metabolites such as alkaloid, flavonoids, terpenoids, saponins, tannins and phenolic compounds which are the important sources of microbicides, antifungal activity and many pharmaceutical compound [12,13]. Many reports on the antiviral, antibacterial, antifungal, anthelmintic, anti-molluscal and anti-inflammatory properties of plants have reviewed. Thus, plant extracts which is a source of natural pesticides can be developed into new biocidal pesticides [14-176]. The goal of our research was intended to determine the composition of fungi on Radish in all agrocenoses in Georgia; study the pathogens and harmfulness of main fungal Radish pathogens and application of the biological method (various extracts of high medicinal plants) against the most common fungi Hyaloperonospora brassicae F. sp. Raphani in Adjara.
MATERIALS AND METHODS
Plant material
19 different plant species Althaea officinalis, Betonica officinalis, Eucalyptus cinerea, Galium aparine, Inula racemosa, Hamamelis mollis, Jasminum officinale, Mentha sylvestris, Mentha longifolia, Panax ginseng, Panax ginseng, Pimpinella anisum, Pyrethrum vulgare, Ricinus communis, Rubia cordifolia, Rubia iberica, Salvia officinalis, Sambucus ebulus and Stevia rebaudiana known for their medicinal value in traditional medicine, were cut into small pieces and dried at room temperature for one week.
Sample collection
This study was conducted between 2017 June and September 2020 in Adjara, Georgia. The objects of research were various varieties of Radish (Raphus sativus) and pathogenic fungi inhabited on Radish.
Isolation of fungi
For the isolation of fungi from Raphus sativus plants, the agar method was applied. Fungal pathogens were responsible for disease isolated from the laves and fruit. From the samples obtained, serial dilutions (1:10) were prepared in test tubes with 9 ml sterile water, adding 1 g of fruit samples until dilutions 10-5, 10-6 and 10-7, of which 50 μL and shaked at 200 rpm for 3 h. Samples collected were seeded by diffusion technique on Petri dishes of 88 mm diameter containing one of the following artificial Potato Dexstrose Aga (PDA) and Czapecks agar medium. After incubation (23°C for 6 days) the number for each fungus was calculated. All fungi were cultured using a single spore technique on PDA and Czapecks medium. After incubation, fungi were identified by their macroscopic and microscopic characteristics.
Identification of fungi
Fungal species identification was based on the morphological characteristics of single spored isolates [18]. Collections of the fungi have been examined by standard light microscopy (Pereval, Cari Zeiss, Jena and Olympus, BX50, Hamburg, Germany). The SEM micrographs have been prepared using a JSM-35 (Japan) SEM microscope.
Disease Assessment
Disease severity it was ratted using a 0 to 9 disease scoring scale; where, 1 = no infections; 2 = 1-10% leaf area infected; 3 = 11-20% leaf area infected; 4 = 21-30% leaf area infected; 5 = 31-40% leaf area infected; 6 = 41-50% leaf area infected; 7=51=60% leaf is infected; 8=61-70% leaf is infected; and 9= 71-100 leaf are infected [19].
Disease severity scores were converted into percentage severity as follows:
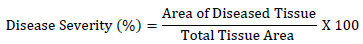
The severity grades were converted into percentage severity index (PSI) for analyses as indicated by Wheeler [20]:
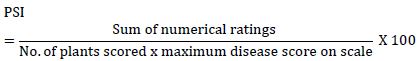
The relative disease severity reduction on untreated and treated plots of the treatment combination was calculated as follows.
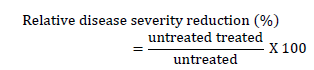
Statistical analysis
The data were analyzed by one - way analysis of variance (ANOVA) and comparisons were carried out for each pair with the HSD Turkey test using SPSS statistical software (SPSS Inc., Chicago, IL, USA). All treatments were carried out in triplicate, and the values are given as means ± standard errors. Differences were considered to be significant when the P-value was less than or equal to 0.05.
RESULTS
Isolation and identification of the microbionts
The microbionts was isolated from Radish tissues showing spot symptom on leaf and root vegetable. On the Radish, 17 species of microbionts (Hyaloperonospora brassicae F. sp. raphani, Albugo candida, Pythium de baryanum, Aspergillus niger, Phytophthora sp., Erysiphe sр., Penicillium sp., Alternaria blight, Alternaria sp., Aphanomyces raphani, Cercospora cruciferarum, C. atrogrisea, Thielaviopsis basicola, Fusarium moniliforme f. sp., Sclerotinia sclerotiorum, Phoma sp., Rhizoctonia solani) were identified as a result of phytopathological and mycological studies conducted in different places of agrocenosis in Georgia.
Formation of the consortium fungus on leaf and root vegetable
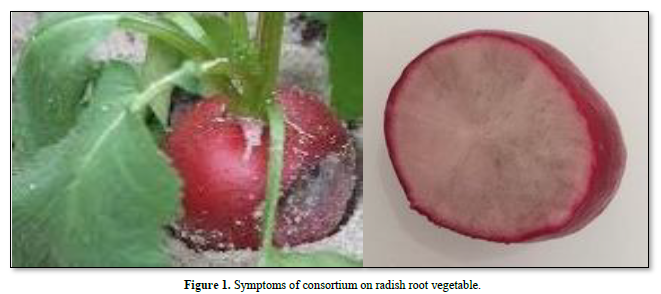
The infected leaf and root vegetable radish can be covered with a multi-colored mycelium which includes 7 species of fungi (Hyaloperonospora brassicae F. sp. raphani, Aphanomyces raphani, Alternaria blight, Alternaria sp., Aspergillus niger, Sclerotium rolfsi, Rhizoctonia solani) in Figure 1.
Observations have shown that the formation of the consortium begins when air and soil temperatures are 25 to-31°С, and an optimum temperature is about 26-27°С. High humidity (89-96%) hastens the formation of the consortium.
Results of the studies showed that among the mycobionts found on radish, the most common, dangerous and harmful are 4 pathogens: Hyaloperonospora brassicae F. sp. Raphani (51%), Rhizoctonia solani (28%), Aphanomyces raphani (23%) and Sclerotinia sclerotiorum (12%) in Figure 2.
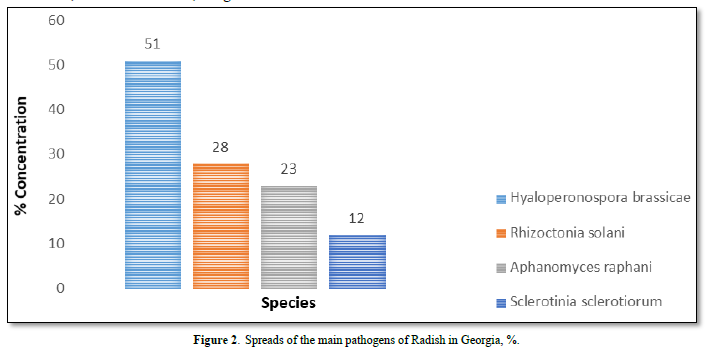
Analysis main pathogen of radish and symptoms diseases
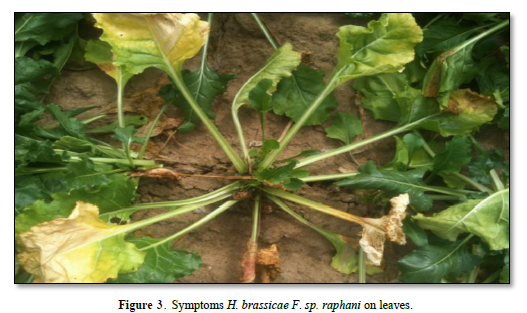
As Figure 2 shows fungi, the main species is Hyaloperonospora brassicae F. sp. rafani Syn.: Peronospora brassicae, Peronospora parasitica. It causes downy mildew. In the past, the cause of downy mildew in any plant in the family Brassicaceae was considered to be a single species Peronospora parasitica. However, this has recently been shown to be a complex of species with narrower host ranges, now classified in the genus Hyaloperonospora, from the perspective of plant pathology, Hyaloperonospora brassicae F. sp. raphani is now the name of the most important pathogen in this complex. The pathogen is more common in seedbeds. In Adjara (favorable for the disease), yellow to pale brown spots develop rapidly into large irregular patches, on the upper surface of the young leaves. A greyish growth occurs on the underside of the leaves and may be present, too, on the upper surface during cool and moist conditions. The diseases areas turn brown and papery in dry weather. Severely affected seedlings are stunted and killed. The older leaves may have a speckled appearance in Figure 3.
As Figure 4 shows symptom on radish root vegetable is a white to gray dusty material. The fungus quickly spreads to completely cover the root vegetable surface. This disease is favored by high humidity, nearly similar symptoms appear in the field and lead to the early death of the leaves; in moist weather the spots grow larger, join together and form large dead patches.

Level of disease incidence and severity of Radish sowings
The distribution area of Downy mildew, level of disease incidence and severity were determined during observation of Radish sowings in Table 1.
As shown in Table 1, the average values of the infected field were high (62,3-54,6%) in the subtropical zone (Xhelvachauri and Kobuleti). The lowest number of infected fields (27.4%) were in the mountainous zone (Khulo). A nearly equal number of infected fields (37.2-34.7%) were determined in Keda and Shuakhevi.
As shown in Table 2, the overall mean of incidence and severity were low (20,1% and 23,0%), especially in the non-irrigated region of Shuahkevi (12,2-15,3%). Drought and hot dry weather during April and May limited the development of Downy mildew and that significantly contributed to the Downy mildew development. In 2020 and 2021 years overall mean of infected fields by Downy mildew were similar (53.2% and 53.4%, respectively) and lower, than in previous years. Also, there was a very low overall mean of incidence and severity of diseases - 20,1% and 23.0% in 2020, 23.7% and 25.3% in 2021, 26.1% and 28.8% in 2022 respectively.
Antifungal activities of the extracts
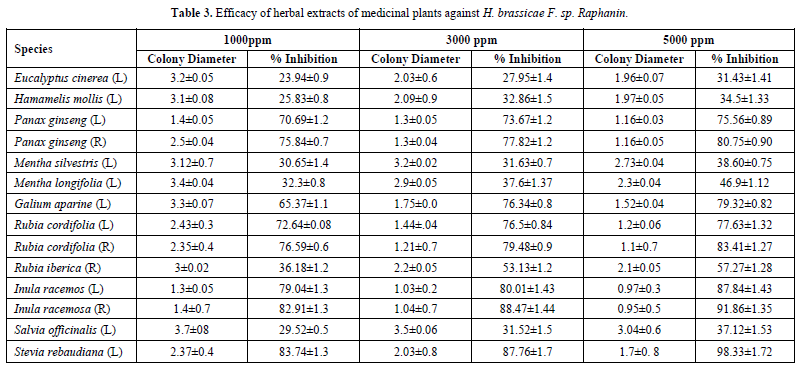
Different concentration i.e. 1000ppm, 3000ppm, 5000ppm of 21 different metabolic extract from 18 different medicinal plant species was tested for their efficacy against H. brassicae F. sp. raphani. Antifungal activity of tested plant extract was investigated at different concentration against H. brassicae F. sp. raphani. The result revealed a highly significant percent inhibition.
The antifungal activity was observed to be dose-dependent i.e. with an increase in the concentration of plant extract percentage inhibition of mycelium growth increases. Antifungal activity of different plant extracts showed significant activity when compared with the leaf/root extract against H. brassicae F. sp. raphani. Root extract of Inula racemosa (R), Rubia cordifolia (R) and Panax ginseng (R) exhibit the highest activity.
Among the 21 extracts evaluated, 5 extract showed mycelia inhibition 80.75-98.33%, 3 showed moderate effect of 75.56 -79.32% and 6 showed least 31,4 % - 57.27% (Table 3). The remaining 7 extracts from leaf extract of Betonica officinalis, Althaea officinalis, Jasminum officinale, Pimpinella anisum, Pyrethrum vulgare, Ricinus communis and Sambucus ebulus showed no antifungal activity against the H. brassicae F. sp. raphani.
This may be due to the lack of antifungal compound in the above mentioned 8 extracts. The percentage growth inhibition of H. Brassicae F. raphani was found maximum with Stevia rebaudiana (98.33%) and Inula racemosa (R) (91.86%). The plant Galium aparine, Inula racemosa (L), Panax ginseng, Rubia cordifolia et al which inhibit the mycelia growth above 75% would probably be important candidates for plants for prevention of against Fungi. The investigation is an important step towards crop protection strategies for antifungal activity against important phytopathogen.
The value means of three replicates ± standard error. The values followed by different alphabets differ significantly when subjected to Tukey HSD at 0.5 subsets; R: Root; L: Leaf.
DISCUSSION
Study confirm that H. brassicae F. sp. raphani in Georgia can be a serious threat to the production of radish. Several phytopathogenic fungi including H. brassicae F. sp. raphani causes a significant decrease in nutritional value. Pathogen has been recorded as the dominant of Radish in Adjara. The use of many agriculturally important synthetic fungicide against this fungus has been banned by the World Health Organization (WHO) in several countries due to its toxicity effect against non-target organisms including human. Thus, there is an urgent need to search for an alternative method for the prevention of biodeterioration of vegetable without any toxicity to the consumer. Plants are rich sources of important organic compounds, pharmaceuticals and pesticide compounds. Many reports on the antiviral, antibacterial, antifungal, anthelmintic, anti-molluscal and anti-inflammatory properties of plants [15,16] have reviewed.
Plant-derived secondary metabolites possessing antifungal activity. There is plenty of biologically active constituent which can be used as potential sources of commercially valuable pesticides which remain to be discovered [21-23]. This may be due to a lack of information on the screening/evaluation of diverse plants for their antifungal potential.
Among the mycobionts found on Radish, the most dangerous and harmful 4 pathogen of which the dominant species is Hyaloperonospora brassicae F. sp. raphani. It was found that effective leaf disease control may prevent root damage because roots are infected by conidia that come from the leaves. Similar findings were made by foreign researchers [3]. Among the 21 extracts evaluated, including 5 extract showed mycelia inhibition 80.75-98.33%, 3 showed moderate effect of 75.56 -79.32% and 6 showed least 31,4 % - 57.27%. The remaining 7 extracts from leaf extract of Althaea officinalis, Betonica officinalis, Jasminum officinale, Pimpinella anisum, Pyrethrum vulgare, Ricinus communis and Sambucus ebulus showed no antifungal activity against the H. brassicae F. sp. raphani. As studies have shown that Stevia rebaudiana, Inula racemosa, Rubia coradifolia and Panax ginseng extracts can be emerged as safe alternatives to replace chemical fungicides against pathogens. This may be due to the lack of antifungal compound in the above mentioned 7 extracts. The percentage growth inhibition of Hyaloperonospora brassicae was found maximum with Stevia rebaudiana (96.78%) and Inula racemosa (R) (92.86%). The plant Inula racemosa (L), Rubia coradifolia, Galium aparine, Panax ginseng, et al. which inhibit the mycelia growth above 75% would probably be important candidates for plants for prevention of against Fungi. Almost similar findings were made by foreign researchers [24-26]. The investigation is an important step towards crop protection strategies for antifungal activity against important phytopathogen.
Based on a general analysis, it is very clearly shown that the investigation is an important step towards developing plant protection strategies for antifungal activity against the important phytopathogen H. brassicae F. sp. raphani. That can be beneficial for farmers and researchers who involve in agriculture. Lastly, by taking into consideration all the information provided in this review, the use of extracts evaluated should be promoted as a valid alternative to pesticides in the era of a green economy that aims at promoting human health and environmental safeguarding.
ACKNOWLEDGEMENT
The authors wish to thank Academician, Prof. Dr. Guram Aleksidze for her valuable advice. In addition, we are grateful to previous students, doctoral and other with whom we have the honor to work for many years.
No Files Found
Share Your Publication :