-
Publish Your Research/Review Articles in our High Quality Journal for just USD $99*+Taxes( *T&C Apply)
Offer Ends On
J L Rodríguez-de la O*, F Pérez-Pérez and M Pérez-Grajales
Corresponding Author: J L Rodríguez-de la O, Department of Plant Sciences, Autonomous Chapingo University Km. 38.5 Mexico-Texcoco. Chapingo. C. P. 56230. Mexico
Received: May 27, 2021 ; Revised: June 04, 2021 ; Accepted: June 07, 2021
Citation: Rodríguez-de la JLO, Pérez-Pérez F & Pérez-Grajales M. (2021) Histology of Microspores of Chile Apple Capsicum Pubescens R and P. and In Vitro Culture. Adv Biotechnol Biopro Res, 1(1): 1-8.
Copyrights: ©2021 Rodríguez-de la JLO, Pérez-Pérez F & Pérez-Grajales M. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Views & Citations
Likes & Shares
Abstract
In plant biotechnology, in vitro culture of gametic or sexual cells, microspores or pollen grains, has been described as a successful tool to accelerate genetic improvement, obtaining haploid, homozygotic plants or pure lines in a short time. In chile apple, Capsicum pubescens R and P. Anthers were sown in vitro, and their cytological analysis, locating the meiotic division stage of microspores or pollen grains. Flower buds with diameters from 2.5 to 4.4 mm were pre-incubated at 4°C, in ascorbic and citric acid at 100 and 150 mg-L-1 for 24 h. Five semisolid culture media (A1, A2, A3, A4 and A5) were used, with Murashige and Skoog (1962) salts (MS), modifying iron and vitamin chelates, sucrose, and L-cysteine, 2,4-dichlorophenoxyacetic acid (2,4-D) and Kinetin (Kin). Anthers, in vitro, were plated, in light and dark, for 70 days. Two differentiation media (R1 and R2) were evaluated with 100% MS salts, glycine, kinetin and myo-inositol. The anthers seeded, coincided with the first mitosis of the microspore, the anthers, formed callus in the media (A1) 100 % EDTA-Fe, 0.40 mg/L-1 thiamine, 3 % sucrose) and (A3) 100 % EDTA-Fe, 0.40 mg/L-1 thiamine, 3 % sucrose, 0. 3 mg/L-1 of 2,4-D, and differentiated pro-embryonic structures in (A3) and (A5) 200 % EDTA-Fe, 0.4 mg/L-1 thiamine, 50 mg/L-1 pyridoxine, folic acid, riboflavin and niacin, 0.3 mg/L-1 2,4-D plus 0.3 mg/L-1 Kinetin, as well as roots in (A1). Light influenced the formation of pro-embryos and roots, in the dark callus. The media (R1) and (R2) favored the formation of pro-embryos.
Keywords: Morphogenetics, Haploid, Anther culture, Organogenetics
INTRODUCTION
Chili bell pepper (Capsicum spp.) is one of the most widely cultivated vegetables in Mexico (SIAP, 2014). It is estimated that about 30 species are native to Mexico: five domesticated and 25 wild [2]. Apple chili (C. pubescens R.), is a species whose fruit is appreciated in America and Mexico for its flavor and high pungency; this species has been increasingly in demand in Mexico and the United States as a result of the diversification of its consumption [3].
Apple chile is a species that presents a certain degree of gametophytic self-incompatibility [4], a phenomenon that somewhat limits the production of inbred lines as hybrid progenitors. One of the main phytosanitary problems faced by this crop is the attack of viruses like chlorotic mosaic virus (CMV), and fungi such as Rhizoctonia solani K. and Phytophthora capsici L. [5] so it is necessary to generate tolerant or resistant varieties to these pathogens. However, the generation of new materials is very low and takes up to five cycles of self-fertilization to achieve a high homozygotic [6], making it necessary to generate new varieties in a short time.
In vitro culture of gametic or sexual cells has been used in plant biotechnology as an alternative to shorten numerous selection cycles and thus obtain pure lines in a single cycle, reducing time and cost in conventional breeding programs [7-9]. This technique can also be applied in genetic studies and mutagenesis, being an important tool in the agronomic and scientific field [8]. In different horticultural species, complete plants have been regenerated from pollen grains or male gametophytes, with a haploid number of chromosomes, which is diploidized to generate pure double haploid lines [10].
The culture of microspores, or anther pollen grains, is the main method for producing haploid plants in chile. There are numerous protocols for the induction of androgenesis in several chile bell pepper species [11-14], however, the information generated as complete protocols for obtaining haploids in apple bell pepper is still limited.
Recent studies on the induction of androgenesis in species of the Solanaceae family converge in that there are several factors that substantially affect the induction of haploid plants. Among the most relevant are the genotype of the donor plant [15-17] and the stage of division and development of microspores in anthers grown in vitro as well as the constitutive characteristics of the culture media and regulators used [9,13].
In this context, the objectives were to determine both the in vitro culture conditions and the meiotic division stage of microspores in anthers of Capsicum pubescens R and P. that favor the morphogenic responses involved in the regeneration of haploid plants.
MATERIALS AND METHODS
The Chile plants used in this research are the result of homogeneous advanced lines of five cycles of self-fertilization, derived from the intraspecific hybrid F1 Grajales ST. Lines, developed in greenhouses, located at an altitude of 2240 m, in the coordinates 19°29'30.37" North Latitude and 98° 51'56.77" West Longitude, the predominant climate is semi-dry temperate with annual maximum and minimum temperatures of 26.2 and 8.2°C respectively. Flower buds were collected between 10:00 and 12:00 h, on one and a half year old cultivated plants.
Approximately 15 plants were sampled in the greenhouse. The diameter of the flower buds was determined with the help of a digital vernier, measuring the middle part of the flower bud. The amount of anthocyanin pigment present in the anthers was also taken into account as another visual indicator of flower bud maturity.
Tissue histology, for the identification of the meiotic division stages of microspores, was performed using the kerosene embedding technique. Subsequently, the slides were observed under a LEICA DM500 optical microscope with a 100 X objective, detecting a total of 3 visual fields per group, establishing for each one the predominant division stage of the microspore. Finally, photographs of each visual field were taken with the microscope's integrated digital camera (ICC50).
According to the review and experimental background, only flower buds containing microspores developed from the uninucleate to mid binucleate stage were used in the following tests. Such flower buds were maintained in a solution of distilled water with citric and ascorbic acid at 150 and 100 mg-L-1 respectively, at a temperature of 4°C for 24 h.
Once the pre-incubation phase was completed, the flower buds were disinfested by immersing them in three different solutions. In the first one, soapy water was used, adding one capful of tween 20 surfactant for each half liter of solution. The second immersion was done with 70% (v/v) ethyl alcohol for 60 s, and the third in 10% (v/v) sodium hypochlorite for 15 min. At the end of each immersion the flower buds were rinsed three times with distilled water.
In a laminar flow hood, the anthers containing the microspores or pollen grains were isolated. With the help of dissecting forceps, the flower bud was grasped and with a scalpel, the sepals and petals were completely removed until the anthers were free.
Finally, the anthers were sown, placing them on the surface of the semigelified medium in petri dishes of 10 cm in diameter (20 ml of medium) during the first 15 days, then they were changed to glass flasks with a capacity of 120 ml (30 ml of medium), with a total of seven anthers per box/flask placed distant from each other.
Five inductive or differentiation media A1, A2, A3, A4 and A5 were used, all with [1] MS salts, 100 % supplemented with 100 mg/L-1 myo-inositol and 7 g/L-1 agar-agar, such media differing in some components and supplements as shown in (Table 1).
After 90 days of in vitro culture on media with promotors or inducers of cell differentiation, the anthers were analyzed considering the responses directed to control the presence of contaminants and levels of tissue necrosis (oxidation), in this way, The clean and viable anthers were subsequently sub cultured to R1 and R2 regeneration media (Table 2), with the aim of promoting morphogenic responses directed to obtaining and forming callus, or other subsequent stages associated with somatic embryogenesis.
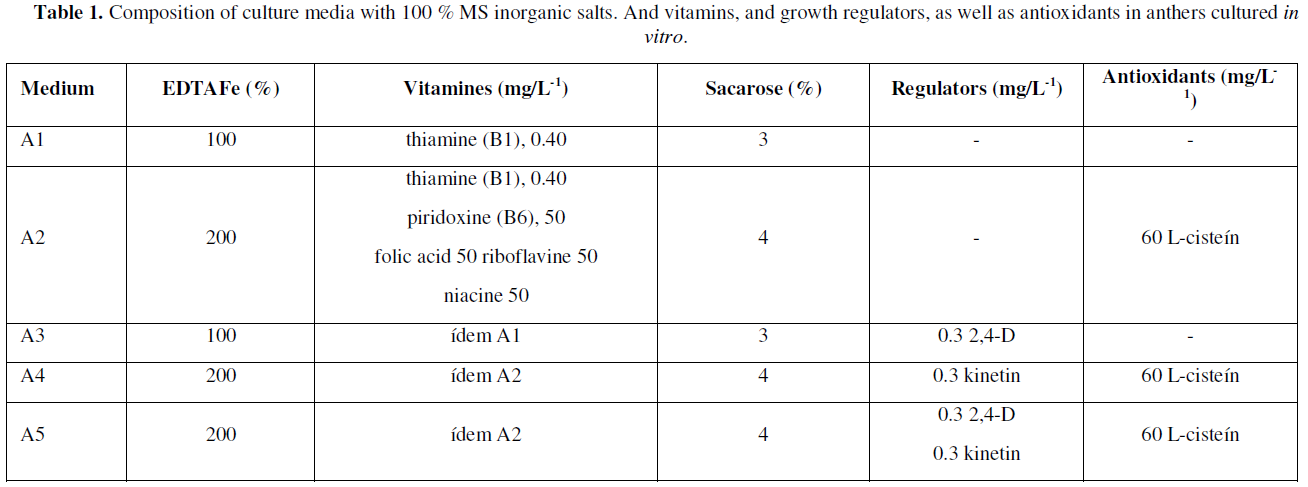
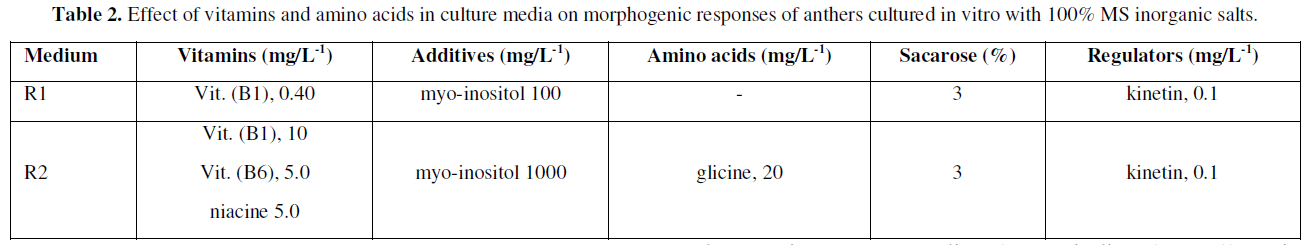
Incubation conditions
The anthers were incubated under two lighting conditions: in the first one they were placed in a dark chamber at 25 °C for one week, and then they were transferred to an illumination condition with a photoperiod of 16 hours light (with 50 watts fluorescent cold white light lamps). Once transferred, the effect of four levels of illumination was evaluated for one week: 14, 28, 28, 29.5 and 40 µmol m-2 s-1 photons, respectively, with a temperature of 26 ± 2 °C. The second environmental condition to be evaluated was to place the anthers directly in the growth room under the initially described dark conditions.
Statistical analysis
For the induction phase, a completely randomized design with 14 replicates (1 replicate = 1 anther) was used. The M x T x L factorial treatment arrangement was carried out for the factors: induction medium, flower bud size and light-darkness with 5, 3 and 2 levels, respectively. The variables evaluated were: number of necrotic (rusty) anthers, number of anthers with callus, number of anthers with pro-embryos and number of anthers that generated root. The significance of the effects of each factor and their interactions was determined by applying hypothesis comparison tests for binomial proportions (0.05). In the regeneration phase, a completely randomized design with 210 replicates (1 replicate = 1 anther) was used for R1 and R2 means, respectively. To determine if there were significant differences for the variable percentage of pro-embryonic anthers, the same test was applied as for the induction medium.
Meiotic division state of microspores
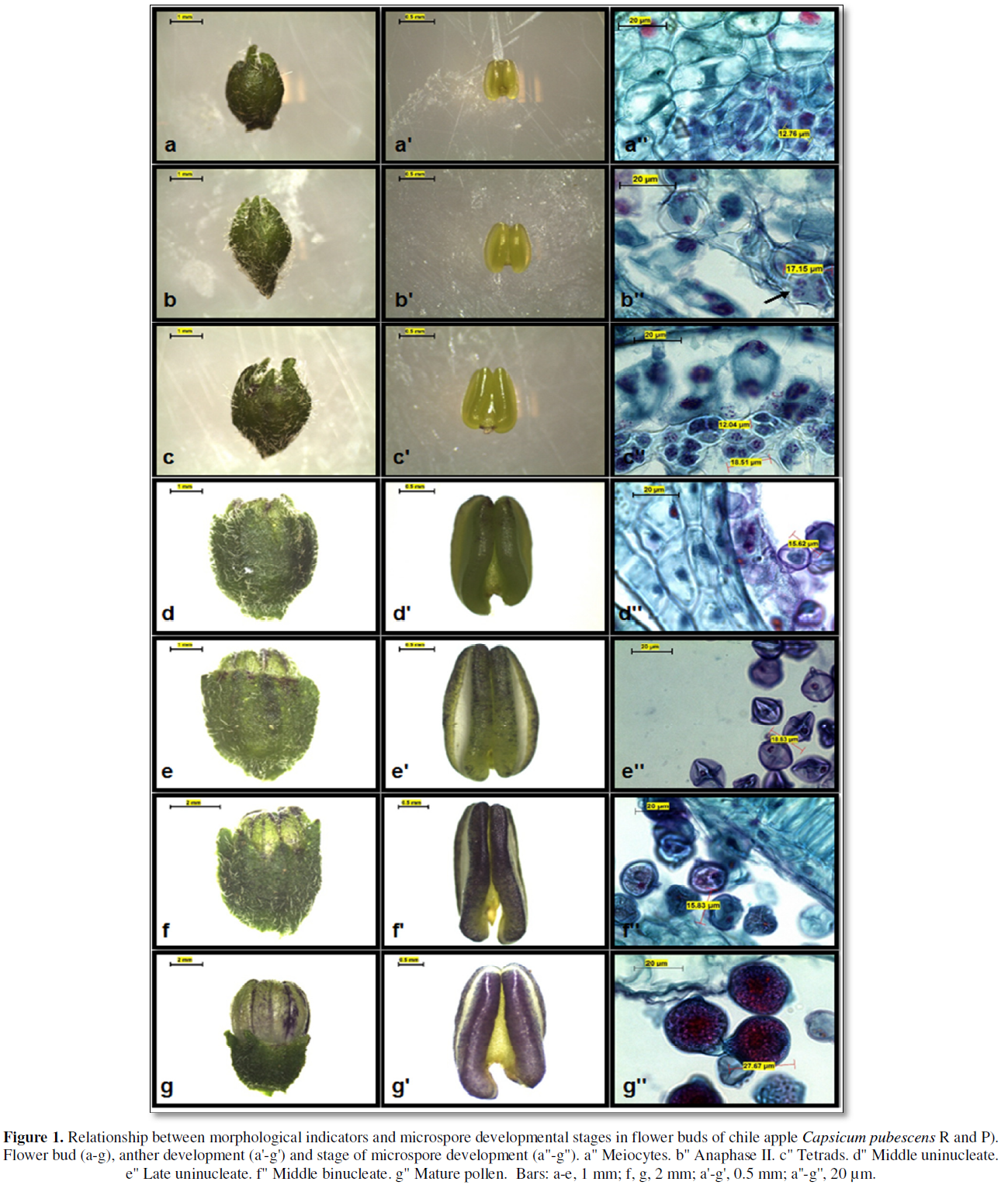
In the results observed in the reductive meiotic division stages of microspores, it was determined that the division stages of microspores in primary stages were strongly associated with the relationship between the size of flower buds, the amount of anthocyanin in anthers and the stage of development of microspores or pollen grains (Table 3). Such indicators have been recognized as morphological characteristics of great utility to determine the stages of cell division in the selection and collection of flower bud size and anthers with microspores or pollen grains (Figure 1), thus ensuring the possibility of obtaining potentially embryogenic responses directly or indirectly (callus) in different chile bell pepper genotypes, which has also been reported by [18-22].
In order to obtain haploid, isogenic or pure plants, the particularity of specifying in a timely manner the stages of reductional division of microspores are a process linked to the meiosis of microspores or pollen grains, and in the case of chile, there are reports that locate the most favorable stages, generally during the first mitosis of the microspore [9, 19,23-25]. In the results of the present investigation, it was found that the primary stages of reductional division of microspores are located in flower buds with diameters between 2.5- and 4.4-mm (Table 3, Figure 1), the above reported, allowed to generate in this way, a rapid selection criterion regarding the collections of flower buds with potential morphogenic response associated with the embryogenesis of this species, or for this chili genotype.
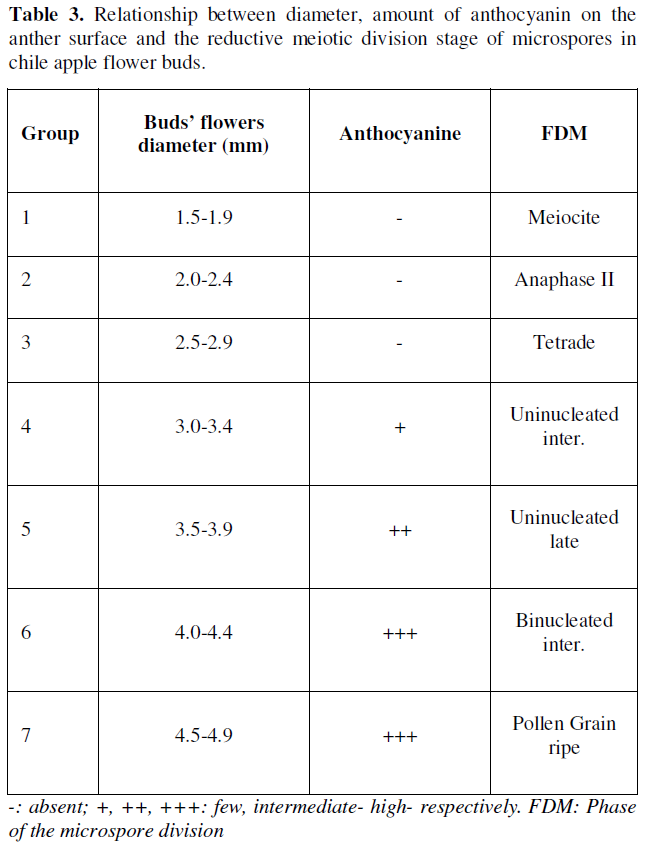
Effect of the Differentiation Media (Induction)
According to the statistical analysis carried out and the test for Z-hypothesis contrasts, no statistically significant differences were found at a p≤0.05/2=0.025 in any of the morphogenic responses obtained; these results show low percentages of morphogenic response in the anthers cultivated in vitro. For this reason, qualitative statistical analyses of the results obtained were carried out as follows.
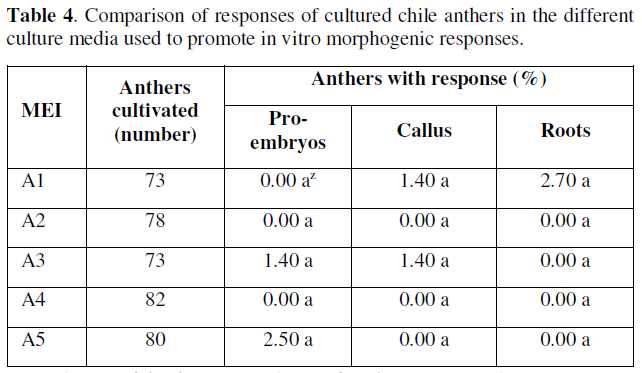
The culture media A5 and A3 (Table 4), were the ones that showed evident morphogenic responses associated to the formation of pro-embryogenic structures, in this way the culture media A5 was the one that had the best percentage of (2.5%), of positive response, which allows us to infer that the culture media with the presence of auxins, were essential in the promotion of the preliminary responses of pro-embryonic or embryogenic type, in this particular, it was observed that the best results were obtained with the joint addition of 2,4-D + kinetin, with vitamins and 4.0 % of sucrose. These results coincide with those reported by [18,26-30], who mention that kinetin and 2,4-D in a 1:1 ratio successfully stimulate the embryogenic responses of microspores in chili cultivated in vitro.
Z Values with the same letter in the same column are not significantly different according to the test statistic for Z-hypothesis contrasts, at a P≤0.05/2=0.025: significant; MEI: Means of induction.
The anthers cultivated in vitro that formed callus were mainly located in media A1 and A3 with 1.4% of response, in the other culture media, the responses towards callus formation were null or very limited. Based on the results obtained for this variable, the percentages in table 4 show that the anthers with responses towards callus formation, the incorporation of iron chelates in the form of EDTAFe at 100% and sucrose at 3% in media A1 and A3 respectively was important, since this allowed us to identify the basic constitution of medium A3 and the presence of auxins responding with a percentage of response equal to medium A1 free of regulators.
This allows us to identify that the responses with low percentages did not influence the presence of sucrose, and the best results that promoted callus formation were the addition or presence of iron chelates in the form of EDTAFe and vitamin thiamine, which favored this response. These results obtained in cultured anthers may also be associated with an osmotic stress of the medium, so that in culture media with the presence of a greater amount of vitamins and sugars promote positive morphogenic responses, the osmotic stability of anthers cultured in vitro has also been reported by [31].
However, it is not ruled out that, in addition to the above, there is the possibility that there is also an endogenous content of regulators and that auxins in the medium may have a significant role both in the formation of callus and subsequently in the in vitro embryogenic responses. As for root formation in the cultured anthers, only medium A1 had a positive result (2.7%), exceedingly even the percentage of responses associated with embryogenesis, although the roots formed can be recognized as mostly differentiated structures and are of adventitious type. This can probably be explained by the content of endogenously participating regulators in the anthers or by the type and concentration of regulators and other constituents of the culture media inadequately present, in addition to other incubation conditions such as unfavorable light and temperatures, which can cause inhibition of cell differentiation in the in vitro morphogenic responses.
Effect of Light
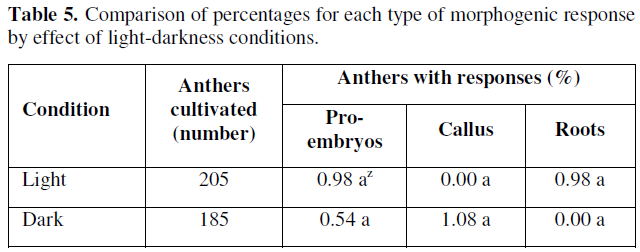
In the observed results, there were no statistically significant differences according to the results and the Z-test. However, it was observed that the presence of light, had a positive effect on the promotion and formation of globular or pro-embryonic type structures and root formation (0.98 %) for both variables, while in darkness the percentage of embryonic response was very limited to a (0.54 %), and the responses of the cultivated anthers was clear and evident in terms of callus formation (Table 5).
Z Values with the same letter in the same column are not significantly different according to the test statistic for Z-hypothesis contrasts, at a P≤0.05/2=0.025: significant.
According to the results presented in Table 5, we can point out that the effect of light favored the differentiation of gametic cells or microspores towards the formation of pro-embryonic structures, coinciding with those reported by [32,33], whose reports mention that incubating anthers from 28.5 to 29 °C under diffuse light conditions increases the frequency of embryo development from microspores cultured in vitro. However, other factors involved are still unknown and more precise studies on the effects of light quality and quantity on morphogenic responses in anthers and microspores cultured in vitro from chile bell pepper are still needed.
Effect of Culture Media on Differentiation
After 10 weeks of anther culture, the anthers were sub cultured to different culture media with different constituents to promote cell differentiation from the cultured anthers. The morphogenic responses were directed towards the promotion of somatic embryogenesis from the microspores or cultured pollen grains contained in the anthers, and according to the results obtained, the anthers did not show notable responses, although in some treatments the appearance of evident pro-embryonic responses (R2) in the cultured anthers could be identified (Table 6).
Z Values with the same letter in the same column are not significantly different according to the test statistic for Z-hypothesis contrasts, at a P≤0.05/2=0.025: significant. MED: Means of differentiation; NP20: Number of pro-embryos at 20 days; NP60: Number of pro-embryos at 60 days.
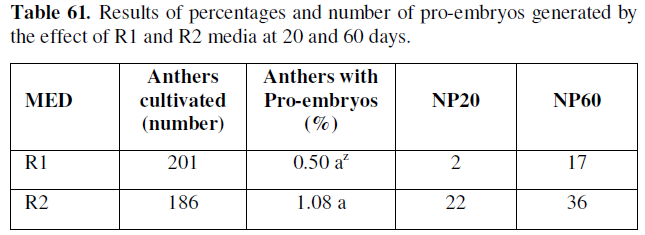
As can be seen in the table above, the percentages of microspores and anthers cultured with de novo pro-embryogenic response, and the number of pro-embryos formed, starts from 20 and 60 days in the treatments of cell differentiation in the experimented phases, thus the R2 medium was identified with 1.08 %, in 22 and 36 respectively.
The responses obtained, allowed to identify that both the addition of vitamins and glycine, as well as increasing the presence of myo-inositol in the treatments experimented allowed to induce cell differentiation, and influenced in a determinant way in the development of the pro-embryonic structures, which coincides with what was reported by [34], pointing out that the presence of myo-inositol, in the culture media, has an important effect in stimulating the morphogenic responses associated with somatic embryogenesis, since it participates in a punctual way in the biosynthetic routes of galacturonic acid, which participate in the formation of several cellular constituents such as ascorbic acid and pectin, in addition to other compounds that intervene in cell division.
As for the addition of vitamins, these are necessary to carry out a series of reactions as catalytic activators, which together with the presence of amino acids such as glycine, is possibly an appropriate source of nitrogen more easily assimilated than that constituted in the basal culture medium. Therefore, we do not rule out the possibility that anthers cultivated in vitro for several weeks are capable of developing other morphogenic responses, and according to the results obtained, R1 and R2 media could be possible promoters or inducers of in vitro responses.
CONCLUSIONS
Flower buds with diameters between 2.5-4.4 mm contain microspores in tetrad to mid binucleate stages, reported as favorable for in vitro culture with the objective of obtaining haploid plants. The inductive media A1 without regulators, A5 with 0.3 mg/L-1 2,4-D plus 0.3 mg/L-1 kinetin and A3 with 0.3 mg/L-1 2,4-D were exclusive for promoting the main in vitro morphogenic responses in apple chili anthers. The presence of light in the cultured anthers favored cell differentiation and the formation of embryogenic structures; on the other hand, the anthers cultured in darkness had little morphogenic response to embryogenesis and only formed callus, which can be cultured later to differentiate the formation of embryogenic structures. The R1 and R2 media were those that responded best in favoring the formation of de novo pro-embryos, so that these structures could later derive complete plants.
ACKNOWLEDGMENTS
The authors of this research sincerely appreciate the support and participation of the technical staff of the Histology Laboratory of the Agricultural Preparatory. University of the Chapingo Autonomous University.
REFERENCES
No Files Found
Share Your Publication :