-
Publish Your Research/Review Articles in our High Quality Journal for just USD $99*+Taxes( *T&C Apply)
Offer Ends On
Reiner Buchhorn*, Christian Willaschek and Christoph Baumann
Corresponding Author: Reiner Buchhorn, Caritas-Krankenhaus Bad Mergentheim, Department of Pediatrics, Uhlandstraße 7, Bad Mergentheim, Germany
Received: February 17, 2022 ; Revised: February 20, 2022 ; Accepted: February 22, 2022 ; Available Online: April 30, 2022
Citation: Buchhorn R, Willaschek C & Baumann C. (2021) The Impact of the Autonomic Nervous System on Inflammation Via the Cholinergic Anti-Inflammatory Pathway - Understanding COVID-19 and Autoinflammation in Children. J Nurs Midwifery Res, 1(1): 1-12
Copyrights: ©2021 Buchhorn R, Willaschek C & Baumann C. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Views & Citations
Likes & Shares
The autonomic nervous system is involved in the regulation of inflammation. Inflammation is indicated by a heart rate increase and decrease of heart rate variability (HRV). We are investigating heart rate variability (HRV) in an acute COVID-19 infection, an 11-year-old boy with multisystemic inflammation syndrome after COVID-19 (MIS-C) and a 16-year-old girl with Postural Orthostatic Tachycardia Syndrome (POTS) after COVID-19. These data were compared with HRV data from children with periodic fever syndromes and severe heart failure to analyze the impact on prognosis.
Result: In acute COVID-19 infection, after a tachycardia up to the fifth day, the heart rate and HRV drop down, which is unusual for infections. As recently published, this "relative bradycardia" is obviously important on prognosis. The MIS-C is characterized by a maximum suppression of the HRV during the monitoring on the pediatric intensive care unit, which is rapidly reversible with intravenous administration of immunoglobulins. The POTS is characterized by a complete collapse of the vagus activity while standing and may be related to elevated G-protein coupled receptor autoantibodies that are also found in our patient with MIS-C. Data from HRV online monitoring on our pediatric intensive care unit demonstrate the high impact of low HRV on prognosis in children.
Summary: The examination of HRV in patients with COVID-19 improves diagnostics and therapy monitoring. The high impact of COVID-19 on the autonomous nervous system opens the opportunity to better understand the interplay between inflammation and the autonomic nervous system and the impact of current therapeutic approaches by using HRV-monitoring.
Keywords: COVID-19, Multisystemic inflammation syndrome (MIS-C), Postural orthostatic tachycardia syndrome (POTS), Heart rate variability (HRV), Autoantibodies
INTRODUCTION
The autonomic nervous system is involved in the regulation of inflammation. This association probably based on the so-called cholinergic anti-inflammatory pathway, as reviewed in 2011 [1]. It has been proposed that efferent splenic vagus nerve signals regulate cytokine production through the nicotinic acetylcholine receptor subunit alpha 7. A current meta-analysis of 51 studies confirmed negative associations between indices of heart rate variability (HRV) - a noninvasive marker of the autonomic regulation - and inflammatory markers [2]. In detail Interleukins (IL-1α, IL-4, IL-12, TNF-α and E-selectin) were inversely associated with heart rate variability measures, including the whole power spectrum of the Fast Fourier Analysis with both sympathetic and parasympathetic associations [3].
In 2001, we show for the first time the association of the neurohormonal activation in infants with severe heart failure due congenital heart disease with elevated cytokines [4-6]. We try to explain the beneficial effects of the beta blocker propranolol in these infants with its immunomodulatory effects. However, we failed to confirm this therapeutic association in a prospective trial with grown-up with congenital heart disease [7] and later we have to publish a severe immunomodulatory complication in an infant with severe heart failure who received Propranolol [8]. However, this therapeutic approach has been currently discussed to combat SARS-CoV-2 hyperinflammatory syndrome [9].
Based upon these experiences, we introduce HRV-online monitoring on our pediatric intensive care unit with the hope for a better understanding of these interactions between the autonomic nervous system and inflammation and to improve the safety of pharmacotherapy.
The current COVID-19 pandemic and the high mortality due to the so-called cytokines storm has suddenly given the forgotten hypothesis of the cholinergic anti-inflammatory pathway a new popularity, if it may open “new” therapeutic approaches [10,11]. So, it made sense to monitor the COVID-19 disease with the HRV analysis and we are now able to describe the unusual bradycardia effect of this illness [12], the collapse of HRV in a an 11-year-old boy with multisystemic inflammation syndrome after COVID-19 (MIS-C) and a Postural Orthostatic Tachycardia Syndrome (POTS) after COVID-19 in a 16-year-old girl.
METHODS
Fundamentals of every HRV data analysis are a group of successive normal RR intervals in sinus rhythm (so-called NN intervals) in a defined period of time of minutes (so called short-term HRV) or 24 h (so-called long-term HRV) (Guidelines 1996). The Time Domain Analysis is done by the processing of the data with simple statistical methods. For frequency domain measures, beat-to-beat fluctuations were transformed to the frequency domain using the fast Fourier transformation algorithm. Spectral power was determined over frequency regions of interest (Table 1).
The children are routinely monitored on the pediatric care unit with Dräger Infinity Monitors™ (Dräger; Germany). For analysis of heart rate variability, we daily export the monitor data to the Pathfinder ™ ECG Software using a network connection. All Holter recordings were reviewed by an experienced cardiologist and were edited to validate the system’s QRS labeling to exclude artifacts. Measures of HRV were calculated employing only normal to normal intervals. The Holter ECGs were analyzed as average values from the entire 24 h of analyzable data. In infants, we use frequency domain measures if the time domain measures have very low values and cannot differentiate the changes of the autonomic nervous system.
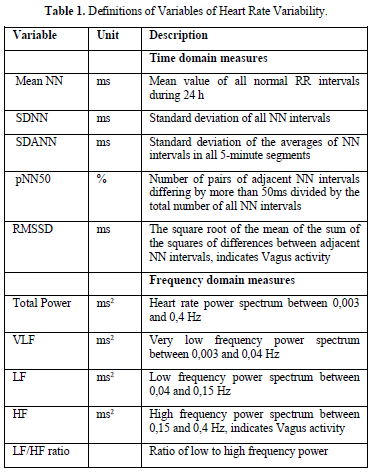
Trend of heart rate variability in the human life cycle (Figure 1)
The standard values for children have been published 1997 by Massin [13] and our own group in 2012 [14]. Standard values for adults have been published 2010 for the United States [15], for Germany in 2005 [16] and 2016 [17]. During lifetime, these standard values show most importantly trends:
Vagus Activity in the Human Life Cycle
Based on thousands of 24-h HRV analysis in children and the adult from literature, we try to interpret these data with respect to the vagus parameter RMSSD (Figure 2). The steep increase of RMSSD within infancy and puberty appears to be a vulnerable phase in the development of the autonomic nervous system (we use the term autonomic imprinting) [18].
and offers a potential window of opportunity for therapeutic interventions. Beside this well-known trends of vagus activity during human life cycle, we found significant differences with lower and higher values that depends on the nutritional state (Anorexia Nervosa with high RMSSD, Obesity with low RMSSD), physical activity (competition sports high RMSSD, Inactivity low RMSSD) and emotional state (Attention deficit hyperactivity disorder/depression/chronic fatigue/posttraumatic stress disorder low RMSSD, the dreaming variant of attention deficit disorder with high RMSSD?)

HRV-MONITORING IN COVID-19
HRV-Monitoring During Acute COVID-19 Infection
Based upon our experience with HRV-analysis, we are able to demonstrate that inflammation in acute COVID-19 in 58-year-old men is associated with unusual lower heart rates and low HRV [11] in contrast to other inflammatory diseases. This observation was confirmed by many other groups [19-22] and seems to be a specific complication in COVID-19 with a high impact on mortality [23,24]. In contrast to these groups who speculate about specific effects of SARS-COV-2 on the sinoatrial node, we anticipate a shared pathophysiology of a brainstem network disruption as shown in other disease like sudden unexplained death in epilepsy [25,26].
Our concept opens new opportunities for risk stratification in the current COVID-19 pandemic in the era of using health technologies in millions of smart watches. Prospectively user-friendly smart watches, mobile ECG devices or chest straps may represent an elegant option in an ambulatory setting to monitor the health risks. Thus, the development of algorithms and advances in machine learning techniques to recognize severe disease like COVID-19 are necessary. A recently published paper used data on 2745 active Fitbit™ users in the USA and Canada who were diagnosed with COVID-19 (active infection, PCR test) [27]. Figure 3 from this publication show the assessment of the physiological measurements with this fitness tracker: Resp Rate=Respiratory Rate, HR=Heart Rate, RMSSD=Heart Rate Variability Parameter that indicate Vagus activity, Entropy=indicating global HRV. There is the expected increase of heart rate, respiratory rate and decrease of HRV within the first two days of COVID-19. In contrast to other infection heart rate drop down within the next 14 days while HRV remained low up to the tenth day. During this time, the life-threatening complication of COVID-19 occur, while the physiological measurements completely recover in most patients who survive.

We recently develop an algorithm to predict life-threatening complications using heart rate variability and the circadian heart rate difference with a special interest on COVID-19, sudden death in epilepsy, children with congenital heart disease and obesity [28]. The algorithm must be proofed in a prospective study to be able to answer the urgent questions of the patients and their parents based on objective data. Otherwise, we must advise all the patients with preexisting chronic disease that they are probably on a higher risk for COVID-19 complications based on single reported cases.
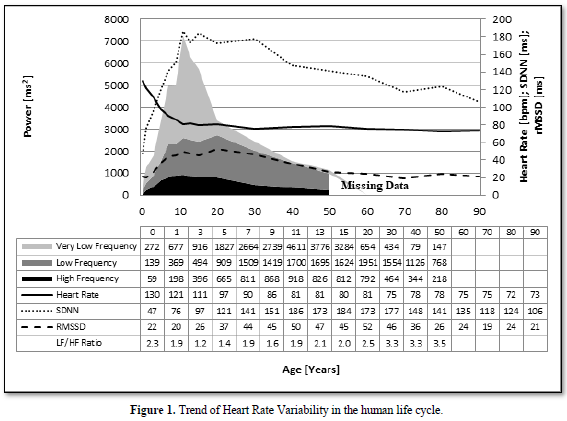
HRV-Monitoring in an 11-Year-Old Boy with Multisystemic Inflammation Syndrome After COVID-19 (MIS-C)
Healthy children who are obviously not at risk from the acute infection can develop the multisystem inflammatory syndrome (MIS-C), who needs pediatric intensive care in 80% of cases with a mortality of 2% [29]. Therapeutical guidelines who based on the experience with the Kawasaki Disease are currently developed [30] and are successful in our current case: It’s a 11-year-old boy whose asymptomatic SARS-COV-2 infection was discovered by chance during a test of contact persons 4 weeks earlier. He developed persistent fever for one week, conjunctivitis and gastrointestinal symptoms. His C-reactive protein (9,4 mg/dl, reference <0,5) and NT-Pro-BNP value (8137 pg/ml, Normwert <85) are highly elevated and decrease after intravenous immunoglobulin therapy (CRP =1,57 mg/dl and NT-Pro-BNP=1108 pg/ml). He completely recovered after a one-week hospital stay. As shown in Figure 4, his heart rate variability indicated by the power spectrum of HRV analysis completely collapsed during the multisystem inflammatory syndrome (MIS-C) and increase to normal values after intravenous immunoglobulin therapy. His coronary arteries are completely normal in the echocardiography.
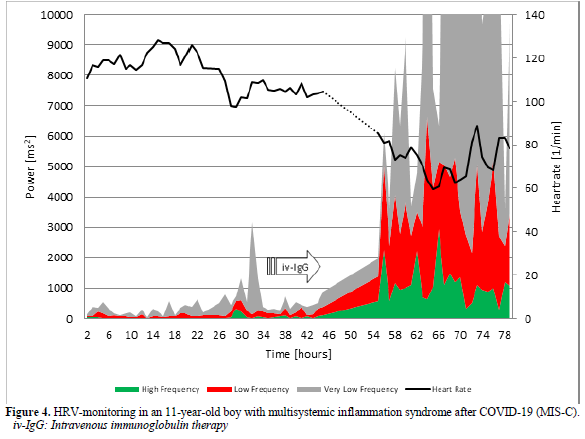
16-Year-Old Girl with Postural Orthostatic Tachycardia Syndrome (POTS) After COVID-19
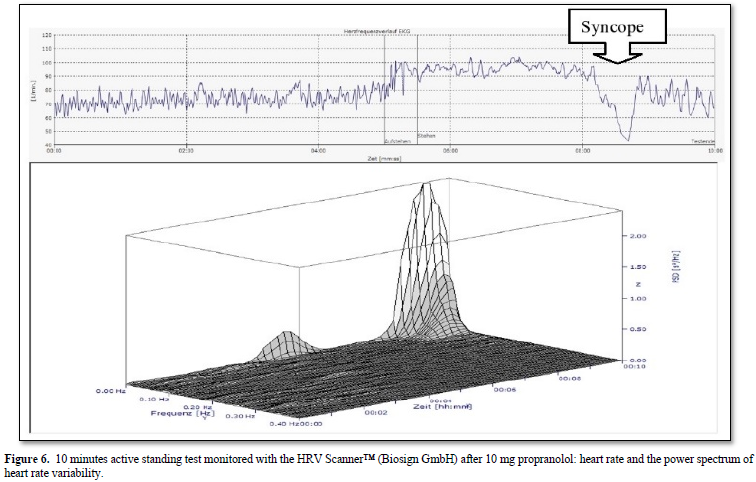
The 16-years-old girl developed dizziness and syncope two weeks after a mild SAR-COV-2 infection proofed by a positive PCR test. The active standing test as shown in Figure 5 show a more than 40 bpm increase of heart rate and the girl had to interrupt the test after one minute. We used the standard therapy for Postural Orthostatic Tachycardia Syndrome (POTS) with low dose propranolol (10mg at 7:00 and 12:00) [31] and the symptoms disappear. However, the girl had severe vasovagal syncope in the control active standing test with sinus arrest > 5 seconds (Figure 6). We must stop propranolol and start midodrine therapy.
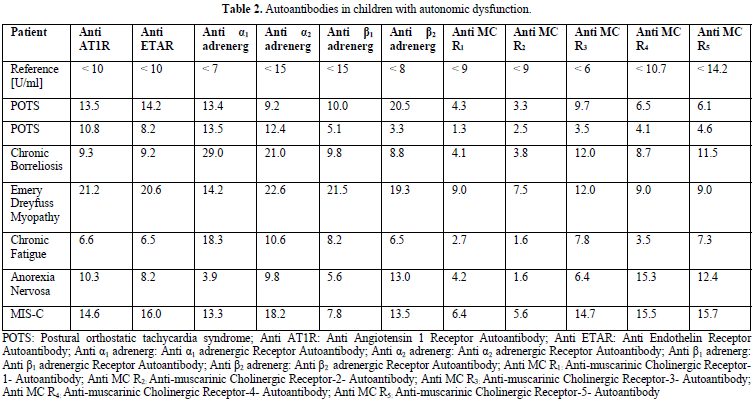
This is the first published case of POTS after COVID-19 in a child after some published cases in adults 32. We are not surprised to anticipate POTS as one correlate of the so called post-acute COVID-19 syndrome if we recently published similar cases of children with POTS after infectious mononucleosis and chronic borreliosis33. In all these children with autonomic dysfunction, we found elevated G-protein coupled receptor autoantibodies as show in Table 2. The current discussion focusing on the role of angiotensin converting enzyme receptors 2 in allowing the viral entry into cells shows, that such receptor networks are important to understand COVID-19.
HRV IN THREE CHILDREN WITH PERIODIC FEVER SYNDROME
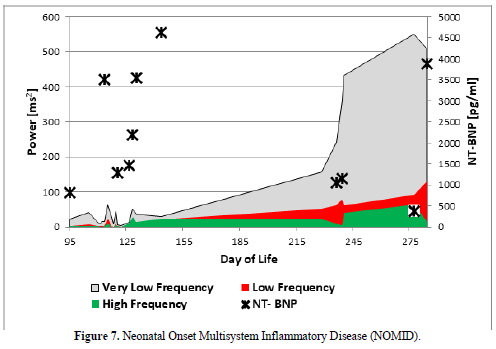
Neonatal Onset Multisystem Inflammatory Disease (Figure 7)
The infant developed a severe inflammatory disease after premature birth called Neonatal Onset Multisystem Inflammatory Disease (NOMID). Our suspected diagnosis was confirmed late by the specialists and we start a specific therapy with the IL-1 antagonist Anakinra at the 215 day of life with a significant improvement of clinical symptoms and autonomic dysfunction. Figure 7 clearly show that low HRV in this infant due to inflammation that was accompanied by very high NT-Pro BNP values, indicating heart failure despite a primary healthy heart. Pro-inflammatory cytokines like Interleukin-1β (8.28 pg/ml), Interleukin-6 (34.6 pg/ml), Interleukin 8 (1500 pg/ml), TNF-α (33 pg/ml) and soluble CD25 (1486U/ml) are significantly elevated. However, after a long in hospital treatment the baby died with one year of age during this therapy in the context of a foudroyant pneumonia.
Hemophagocytic Hymphohistiocytosis in an Infant with Severe Heart Failure
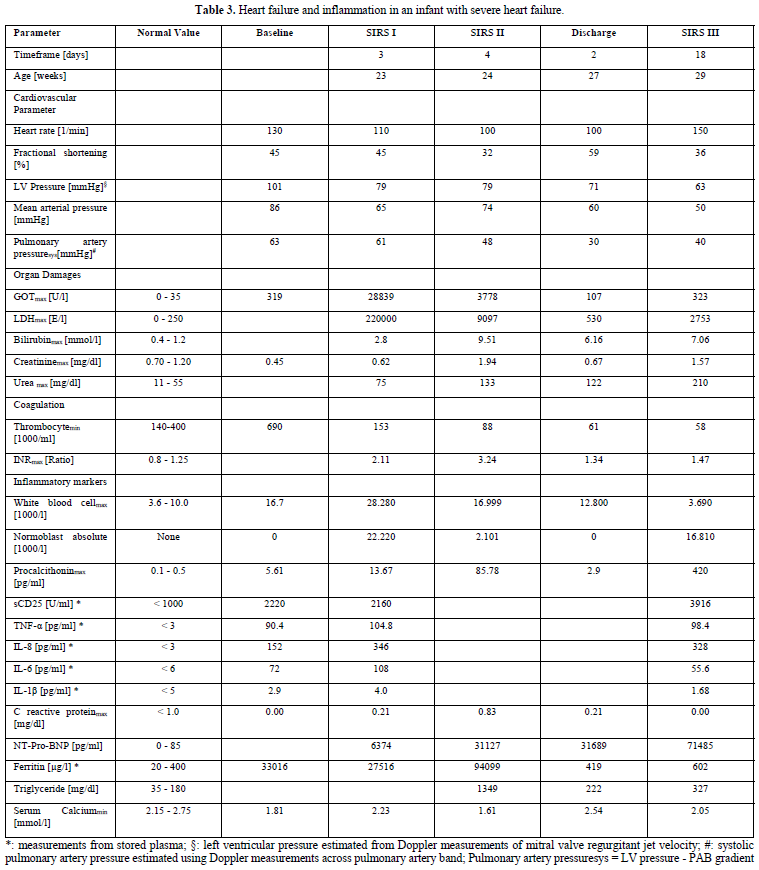
The boy was born with Down`s syndrome and an unbalanced, left dominant atrioventricular septal defect with aortic coarctation. Despite coarctation repair and pulmonary artery banding, he suffers from intractable heart failure and fever of unknown origin indicated as systemic inflammatory response syndrome (SIRS) in Table 3. He remained in heart failure with high NT-Pro-BNP and cytokine values despite multiple therapeutic and diagnostic efforts over 5-month intensive care to stabilize his cardiopulmonary status. He received a low dose propranolol which resulted in dramatic immunomodulatory effects (SIRS I in Table 3). Immunoactivation was indicated by high procalcitonin- and cytokine release, normoblastosis and highly elevated Ferritin values. Immunoactivation leads to a hepatic/splenic compromise as indicated by high lactate dehydrogenase and alanine aminotransferase levels probably caused by macrophage activation. A therapeutic switch to β1 receptor blocker metoprolol appeared to be instrumental in hemodynamic improvement and allowed the discharge from hospital. The heart rate variability remained very low and the infant died of cause inflammatory reactivation (SIRS III).
The autopsy results revealed hemophagocytic lymphohistiocytosis and a very small spleen probably after autolysis. The interactions between the adrenergic system and the cytokine network in this highly activated inflammatory state are discussed in our publication (Table 3) [8].
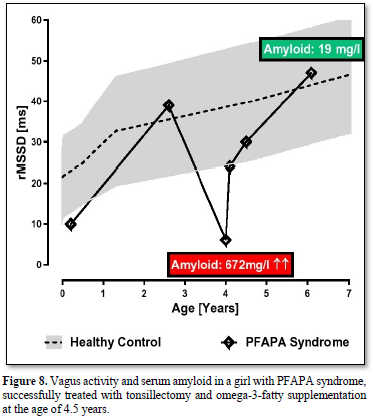
PFAPA Syndrome (Figure 8)
Periodic Fever, Aphthous Stomatitis, Pharyngitis and Cervical Adenitis (PFAPA) syndrome is the most common auto inflammatory disorder among children and an important differential diagnosis in children presenting with recurrent fever episodes [34]. There is considerable heterogeneity in the treatment and a lack of evidence-based treatment.
We report about a girl with periodic fever syndrome beginning at the age of 3.5 years. Fever episodes usually last 3-6 days with a striking regularity and are associated with one or more of the cardinal symptoms aphthous stomatitis, pharyngitis, tonsillitis, and cervical adenitis. During the episodes, the girl has elevated inflammatory variables such as CRP and serum amyloid A. Up to the age of 4.5 years, she had five hospital stays in different hospitals and received different antibiotic treatments of cause the diagnosis sepsis. We decide for tonsillectomy together with a supplementation of 1g omega-3-fatty acids. The girl recovered and within the next two years the fever episodes slowly disappear, and she need no further hospital stay. The very low HRV indicated by the parameter RMSSD at the years of 4 years slowly increase to normal values at the age of 6 years (Figure 8). The highly elevated serum amyloid decrease to normal values (672mg/l to 19mg/l).
HRV Online Monitoring of Life-Threatening Diseases in Pediatric Care Units
The vagus activity, indicated by the HRV-parameters RMSSD and High Frequency Power (HF-Power) are low in nearly all children with severe somatic diseases, most of all in patients with heart failure and inflammation. As recently published in preterm, very low vagus activities indicated by High Frequency Power values below 20 ms2 indicate a high risk of necrotizing enterocolitis, a life-threatening inflammatory disease [35].
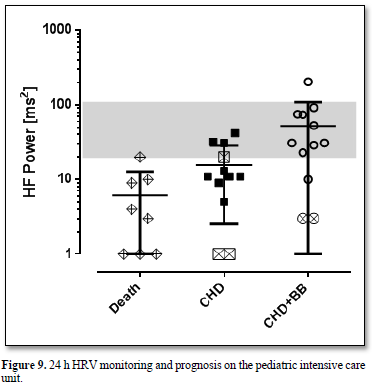
All infants who died on our intensive care unit within the last ten years from cardiac and non-cardiac reasons had such low High Frequency Power values below 20 ms2 more than 1 week before death (Figure 9). We observed a fluent overlap between heart failure and inflammation in these children as illustrated in the case 3.1. and 3.2.
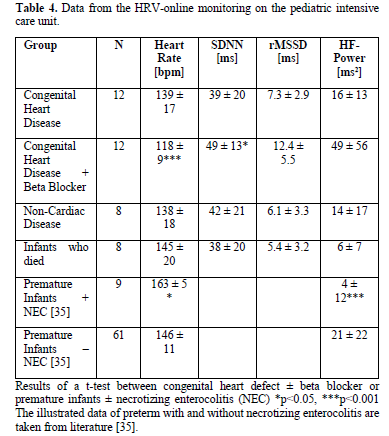
The measured HRV data are illustrated in Table 4, showing the significant difference of infants with severe heart failure before and after beta blocker treatment and the very low HRV in the infants who died.
Legend Figure 9 All infants who died on our pediatric intensive care unit from cardiac and non-cardiac reasons (group Death) had low HF power values below 20 ms2 more than 1 week before death. Infants with severe heart failure due to congenital heart defects had such low HF-power values prior to propranolol (group CHD). HF Power improves during beta blocker treatment (group CHD+BB) but remained low in the two children with very low HF Power who died some month later. One infant with trisomy 18 and single ventricle received no medical therapy and died in a palliative setting.
DISCUSSION
Inflammation is indicated by the heart rate increase and decrease of HRV! A recent meta-analysis shows that the standard deviation of R-R intervals (SDNN) and the power in the high frequency band of HRV showed the strongest and most robust associations with inflammatory markers [2]. As shown in our case collection, severe heart failure ± inflammatory syndromes in infants are associated with a decrease of the power in the High Frequency band of HRV below 20 ms2 and have a very high mortality risk. Furthermore, severe heart failure due to a congenital heart defect may be the cause of inflammation as demonstrated in case 3.1. Conversely, a congenital inflammation syndrome can be the cause of heart failure indicated by very high NT-Pro-BNP values (case 3.2.).
We routinely monitor the effect of pharmacotherapy in pediatric heart failure [36] and other life-threatening diseases on our pediatric intensive care unit on heart rate variability. As shown in many publications, we successfully use heart rate variability monitoring as a therapeutic target and surrogate parameter for the control pharmacotherapy as proposed 18 years ago [37].
The current COVID-19 pandemic and the high mortality due to the so-called cytokines storm has suddenly given the forgotten hypothesis of the cholinergic anti-inflammatory pathway a new popularity and probably open “new” therapeutic possibilities. We are now able to publish the therapeutic effect of intravenous immunoglobulin therapy on HRV in an 11-year-old boy with multisystemic inflammation syndrome after COVID-19 (MIS-C). Furthermore, we could demonstrate that inflammation in acute COVID-19 is associated with unusual lower heart rates and low HRV [11]. This observation was confirmed by many other groups [19-22] and seems to be a specific complication in COVID-19 with a high impact on mortality [23,24]. In contrast to these groups who speculate about specific effects of SARS-COV-2 on the sinoatrial node, we anticipate a shared pathophysiology of a brainstem network disruption as shown in other disease like sudden unexplained death in epilepsy [25,26].
We recently develop an algorithm to predict life-threatening complications using heart rate variability and the circadian heart rate difference with a special interest on COVID-19, sudden death in epilepsy, children with congenital heart disease and obesity [28]. The algorithm must be proofed in a prospective study to be able to answer the urgent questions of the patients and their parents based on objective data. Otherwise, we must advise all the patients with preexisting chronic disease that they are probably on a higher risk for COVID-19 complications based on single reported cases.
We can further demonstrate how to diagnose Postural Orthostatic Tachycardia Syndrome (POTS) in a 16-year-old girl with a simple active standing test as a correlate of the so called post-acute COVID-19 syndrome and a potential therapeutic target. We currently begin to understand how elevated G protein-coupled receptor antibodies are associated with the Postural Orthostatic Tachycardia Syndrome (POTS) [38] and COVID-19 [39].
The high impact of COVID-19 on the autonomous nervous system [40] open the opportunity to better understand the interplay between inflammation and the autonomic nervous system and the impact of current therapeutic approaches by using HRV-monitoring.
No Files Found
Share Your Publication :