-
Publish Your Research/Review Articles in our High Quality Journal for just USD $99*+Taxes( *T&C Apply)
Offer Ends On
Kishore Kumar R*, Annu Kaushik and Haridas
Corresponding Author: Kishore Kumar R, Notre Dame University, Perth, Australia.
Received: March 18, 2024 ; Revised: March 29, 2024 ; Accepted: April 01, 2024 ; Available Online: April 15, 2024
Citation: Kumar KR, Kaushik A & Haridas. (2024) A Retrospective Study to Evaluate the Rates of Hospital Acquired Infections amongst Mothers and Infants in the Tertiary Maternity Unit: Insights from a Hospital Network in India. J Nurs Midwifery Res, 3(1): 1-9.
Copyrights: ©2024 Kumar KR, Kaushik A & Haridas. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Views & Citations
Likes & Shares
Background: Hospital-acquired infections (HAIs) pose a significant public health concern, impacting patient safety, healthcare systems, and society. Implementing effective infection prevention and control (IPC) programs is crucial in mitigating the incidence of HAIs.
Methods: This retrospective study analyzed laboratory-confirmed HAIs in maternity wards and neonatal intensive care units (NICUs) in the Cloud nine Hospital Network (CHN) over 15 years (2008-2023). Data were collected through active surveillance methods, including patient records and culture testing. Incidence rates and incidence density of HAIs (central line-associated bloodstream infections (CLABSIs), catheter-associated urinary tract infections (CAUTIs), surgical site infections (SSIs), and ventilator-associated pneumonia (VAP)) were calculated, and comparisons were made with national and international data.
Results: A total of 2,29,094 females and 15,541 neonates were included in this study. The overall incidence of HAIs in CHN was 7.79 per 1000 patient days. Average incidence rates over 15 years were SSIs: 0.8/100 surgeries; VAP: 1/1000 ventilator days; CLABSI: 2.2/1000 central line days; and CAUTI: 0.1/1000 catheter days. Notably, the incidence rates of SSIs, CAUTI, VAP, and CLABSI were relatively low compared to international rates of both, the International Nosocomial Infection Control Consortium (INICC) and Center for Disease Control and Prevention-National Healthcare Safety Network (CDC-NHSN) reports, except for those of the CLABSI, which was lower than that of the INICC, but higher than that of the CDC NHSN. Moreover, the study results are indicative of the influence of COVID-19 pandemic on HAI rates, with a reduction observed in SSI, VAP, and CAUTI rates, while CLABSI rates increased.
Conclusion: This study highlights the low incidence of HAIs in the maternity wards and NICUs of CHN over 15 years and emphasizes the importance of specialized targeted interventions and robust infection prevention and control (IPC) measures. These findings offer guidance for healthcare facilities to evaluate their IPC measures and enact specific interventions to minimize HAIs and enhance overall patient safety.
Keywords: Hospital-acquired infections (HAIs), Ventilator, urinary tract infections, Neonates, Ventilator associated pneumonia (VAP), Lower segment cesarean section, Nosocomial infections
INTRODUCTION
According to World Health Organization (WHO), Hospital-acquired infections (HAIs), previously known as nosocomial infections (NIs), refer an infection contracted by a patient who was admitted for a condition unrelated to the infection itself. It encompasses infections that manifest in patients within a hospital or another healthcare facility (HCF), even if the infection was not present or in its early stages at the time of admission. This definition extends to infections acquired during the hospital stay but becoming apparent after discharge. Additionally, it encompasses infections contracted by the staff of the facility as part of their occupational exposure [1,2]. Typically, these infections manifest within 48-72 h of hospitalization or within 10 days following discharge [3]. The worldwide HAI prevalence is reported at 0.14%, with a gradual annual increase of 0.06% [4]. In developing countries like India, HAIs are a major challenge for patients [5]. Compared to HAI rates of 4.5 to 7.1 per 100 patients in the Western world, low and middle-income countries (LMICs) face a higher prevalence of 15.5 per 100 patients. Low middle-income countries also see a threefold increase in HAI rates within Intensive Care Units (ICUs) compared to developed nations [5,6]. The World Health Organization (WHO) states that 1 in 10 patients affected by HAIs succumbs to the infection, but effective infection prevention and control (IPC) programs can reduce HAIs by almost 70% [2]. However, there are significant knowledge gaps regarding IPC implementation in neonatal care [7]. Moreover, a recent study presents proof of inadequate compliance with IPC in maternal wards stemming from insufficient documentation or the 'invisibility' of HAIs, low prioritization of IPC tasks, absence of IPC goals and resources, discretionary application of guidelines, and challenges related to communication [8]. HAIs are a significant global health threat, especially in critical care units, causing increased mortality, morbidity, and financial burdens on communities [9,10]. The Centers for Disease Control and Prevention (CDC) broadly categorizes types of HAIs as central line-associated bloodstream infections (CLABSI), catheter-associated urinary tract infections (CAUTI), surgical site infections (SSI), and ventilator-associated pneumonia (VAP) [10,11]. Each type of HAI has specific risk factors and preventive measures to reduce their occurrence. HAIs in neonatal care pose a significant challenge, leading to morbidity, mortality, prolonged hospitalizations, heightened risk of severe neurodevelopmental disorders (especially in premature infants), and increased medical costs [12,13]. The incidence of HAIs in neonates is about 7.5 times higher than in adults [2]. In India, many studies show a high incidence of neonatal bloodstream infections, often linked to preterm labor [14,15]. Recent research emphasizes that factors like low birth weight (LBW), prematurity, hospital stay duration, central venous catheter usage, and mechanical ventilation are significantly associated with higher rates of HAIs in neonatal intensive care units (NICUs) [16]. Similarly, a study has reported barriers to effective IPC in maternity wards to be the efficacy of training, insufficient accessibility to personal protective equipment, inadequate adherence to hand hygiene practices, and outdated procedures for reprocessing reusable medical equipment [17]. Furthermore, the occurrence of HAIs in hospitalized neonates presents a multifaceted challenge, stemming from the potential transmission of microorganisms among patients through healthcare workers (HCWs) and caregivers, such as parents and family members. Additionally, contamination of the hospital environment and equipment contributes to the spread of infections [7]. Some of the maternal factors that can predispose neonates to infections are premature rupture of membranes, maternal fever within two weeks prior to delivery, meconium-stained amniotic fluid (MSAF), foul-smelling liquor, and instrumental delivery [18]. It has been reported that in many LMICs, most neonatal units may lack a formal monitoring mechanism or a systematic strategic response to contain HAIs [19]. To address these concerns effectively, it is essential to implement surveillance systems focused on endemic HAIs with standardized definitions and establish evidence-based best practices for IPC systems. With the promotion of institutional deliveries, there has been an increase in hospital births, exposing patients to the risk of HAIs. To mitigate adverse outcomes resulting from HAIs, it is crucial to establish, implement, and regularly audit IPC measures in clinical establishments [20]. This study aims to assess the existing evidence base and provide insights to inform future actions in reducing the incidence of HAIs using the Cloudnine Hospital Network (CHN; a chain of maternity, children’s, and fertility hospitals) as a prototype.
METHODS
Study Design
This study was a retrospective analysis of laboratory-confirmed HAIs in patients admitted to the maternity ward and neonates in the NICUs in different hospitals of CHN. The CHN encompasses 23 facilities located in 12 cities throughout India. Patients admitted between 2018 and 2023 across different centers were included in the study. The patient population encompassed pregnant women undergoing lower segment caesarean section (LSCS) or normal vaginal delivery (NVD), as well as non-pregnant women who underwent laparoscopic surgeries, hysterectomies, tubal ligation, and other gynecological surgeries. Additionally, neonates admitted to NICUs were included in the study. The occurrences of VAP and CLABSI were evaluated in neonates admitted in the NICU. The study was temporarily halted due to the severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2) pandemic, which significantly reduced admissions.
The inclusion criteria for this study were:
The exclusion criteria were:
All NICU-hospitalized neonates (≥48 h) were monitored until NICU discharge. Infections were considered as a HAI, if occurring ≥48 h post-birth/admission. CLABSI and VAP were recorded only for NICUs.
Ethical Considerations
Since this was a retrospective study, the ethical committee gave a waiver for consent.
Data Collection
The data was collected on birth details, admission/discharge dates, gender, gestational age, birth weight, delivery type, admission diagnosis (preterm birth, twin pregnancy, respiratory distress), invasive device exposure (central/peripheral catheter days, mechanical ventilation days), antimicrobial usage, infection site, onset date, and isolated microorganisms. Information regarding the occurrence of HAIs was obtained from the annual patient records during the study period. The data were collected through active surveillance methods, including both laboratory and patient-based approaches. The IPC team was responsible for tracking, enrollment, and assessment of HAIs. Information on each patient who had contracted a HAI was recorded on a standardized data collection form: patient information (including, age, and gender), results of microbiological cultures, preexisting medical conditions, factors contributing to HAIs, medical procedures during hospitalization, the purpose of admission, and administered treatments. All HAIs were confirmed through culture testing.
Definitions
The rates of HAIs were assessed using two distinct calculations. Firstly, the occurrence rates of HAIs were derived by dividing the total number of patients with HAIs by the overall number of patients admitted to the hospital throughout the observed period and then multiplying by 100 to express it as a percentage. Next, the occurrence rate of HAIs was calculated by dividing the overall number of documented HAIs during the observed timeframe by the total number of patient days. The result was then multiplied by 1000 to present it as occurrences per 1000 patient days.
The rates of occurrence for device-associated HAIs, specifically VAP, CLABSI, and CAUTI, were calculated per 1000 device days. This was achieved by dividing the total count of device-associated HAIs by the corresponding total number of specific device days and then multiplying the outcome by 1000.
Statistical Analyses
Statistical analyses were carried out on Microsoft 365 Excel. Data represented on a qualitative scale are presented as sample counts and percentages.
RESULTS
Patient Population
Over the period from 2008 to 2023, surgeries were performed on a total of 2,29,094 patients across various hospitals. Additionally, 15,541 neonates were admitted to the NICUs. Figure 1 illustrates the year-wise admissions of patients from 2018 to 2023 undergoing surgeries and those admitted to NICUs.
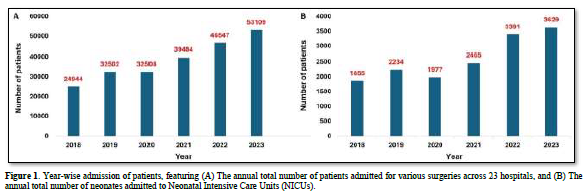
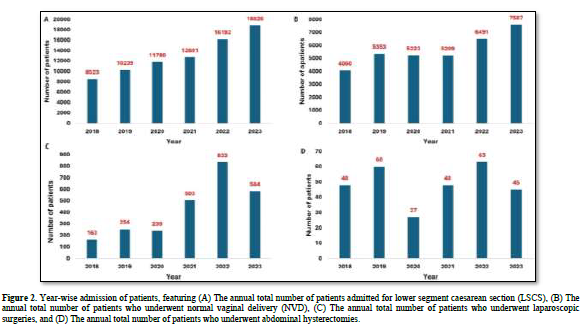
Moreover, 78,251 patients underwent LSCS, while 33,923 had experienced normal vaginal delivery (NVD). Among females admitted for various surgeries, 2,576 underwent laparoscopic surgeries, and 291 underwent abdominal hysterectomies.
Figure 2 depicts the year-wise distribution of patients in these categories from 2018 to 2023.
HAI prevalence
During the study period, 1906 laboratory-confirmed HAI episodes occurred in 2,44, 634 aged between 17.6 to 37.7 years, with a mean age of 30.43 years. The occurrence of HAI was 7.79 per 1000 patient days. No deaths were reported. The average incidence rates over 15 years for different HAIs were as follows: SSIs (including SSI after lower segment cesarean section [LSCS] and other types of SSIs) had an incidence rate of 0.8 per 100 surgeries and CAUTI had an incidence rate of 0.1 per 1000 catheter days. VAP and CLABSI were solely recorded from the NICUs, with an incidence rate of 1 per 1000 ventilator days and 2.2 per 1000 central line days, respectively (Table 1).
The study identified the highest yearly average incidence rates for these HAIs as follows: 0.8/100 surgeries (2021-2022) for SSIs, 0.7 per 1000 ventilator days (2019-2020) for VAP, 3.2 per 1000 central line days (2020-2021) for CLABSI, and 0.7 per 1000 catheters days (2019-2020) for CAUTI. The 5-year trend from 2018 to 2023 for different HAIs in maternity ward and NICU is depicted in Figure 3.
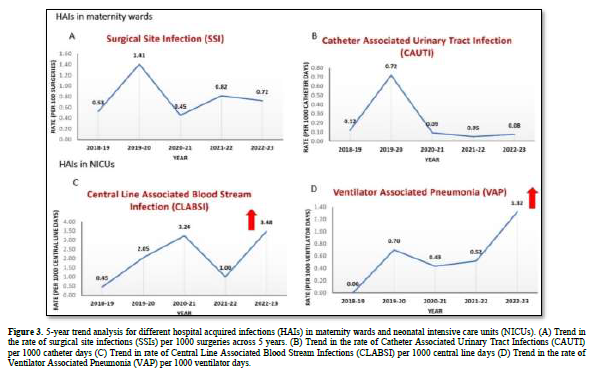
Initially (2018-2020), the trend analysis indicates an increase in all HAIs. However, during the COVID-19 pandemic (2020-2022), there was a decrease in the rate of SSI, VAP, and CAUTI incidence. Conversely, there was an increase in the rate of CLABSI incidence during this period. An overall increase in the incidence of SSI, VAP, and CLABSI were observed across the years; however, the rates of CAUTI incidence decreased across the years.
DISCUSSION
Hospital-acquired infections are a pressing public health concern with grave implications for patients, healthcare systems, and society at large especially in a country like India. In NICUs, HAIs, particularly device-associated infections (DAIs) like VAP, CLABSI, CAUTI, and device-associated ventriculitis, present a significant challenge [23]. Notably, VAP and CLABSIs are the most prevalent DAIs [24,25]. In this study, reported rates of SSI, VAP, CAUTI, and CLABSI in the CHN were significantly lower than those reported by the International Nosocomial Infection Control Consortium (INICC) and the Center for Disease Control and Prevention-National Healthcare Safety Network (CDC-NHSN). The rates as reported by INICC for SSI, CAUTI, VAP, and CLABSI are 0.7, 3.16, 10.7, and 4.8 [26]. Similarly, the rates as reported by CDC-NHSN for SSI, VAP, CAUTI, and CLABSI are 1.8, 0.2, 0.8. and 0.6 [26,27]. Reported HAI prevalence rates across studies are as follows: incidence of SSI after LSCS is reported at 10.3 per 100 surgeries in north India [28]. An international study reported a point prevalence of 2.1% for VAP and 1.2% for CLABSI in NICUs [29]. In India, the CLABSI rate is reported as 16.25 per 1000 central line (CL) placements in NICUs [5]. A meta-analysis in developing countries revealed a median CLABSI rate of 18.7 per 1000 CL days in NICUs [5,30]. Incidence of VAP is found to be 3 per 1000 ventilator-days in NICUs [31]. In a recent study from a tertiary care center in India, the reported comprehensive CAUTI rate is 7.03 cases per 1000 catheterizations [32]. The current study reported HAI incidence rate to be 7.78 per 1000 patient days. Further categorizing as per different HAIs, the SSI prevalence rate was 0.8 per 100 surgeries, notably lower than rates in other studies [32] (7.03 per 100 surgeries in Sushitha or 1.8 per 100 surgeries in CDC-NHSN). Interestingly, INICC data aligned closely, reporting a rate of 0.7 per 100 surgeries. Regarding CAUTI, the prevalence rate was remarkably low at 0.1 per 1000 catheter days, negligible compared to other studies [32]. (Sushitha CDC-NHSN and INICC data). Notably, this study focused exclusively on maternity wards, lacking comparable studies. Moreover, this study found low rates for VAP and CLABSI, at 1.0 per 1000 ventilator days and 2.2 per CL days, respectively. These rates were notably lower compared to a similar Indian study [5,33] (Mehta and international rates by the INICC. However, they were slightly higher than CDC-NHSN. VAP and CLABSI rates were only recorded from NICUs. Many risk factors are associated with HAIs in neonates. Studies emphasize a higher prevalence of HAIs in LBW neonates, with 60.8% occurring in those weighing less than 1000 g. Notably, HAIs were observed across all birth weight (BW) classes [34]. Thus, monitoring all BW classes in NICUs following the NHSN protocol is crucial [34,35]. Indian studies also underscore the vulnerability of LBW neonates to HAIs. Recent research highlights that very preterm neonates with a BW below 1500 grams and NICU stay exceeding 25 days have a higher likelihood of developing CLABSI [5]. Additionally, male gender, extended hospitalization, and cesarean delivery are statistically significant risk factors for VAP occurrence in NICUs [31]. Despite efforts, substantial reductions in neonatal VAP rates have not been achieved [36,37]. The lack of a precise neonatal VAP definition and diagnostic test contributes significantly [38]. This absence impacts clinical decision-making and the application of VAP as a quality metric [36]. Furthermore, the frequency of CAUTI is directly correlated with catheterization duration [32]. Reducing CAUTI involves minimizing catheter procedures, shortening catheterization duration, implementing aseptic precautions, and administering appropriate prophylactic antibiotics [32]. In the current investigation into neonatal sepsis cases, metabolic diseases, often mimicking sepsis, were identified in 0.02% of cases over the hospital's 16-year history, emphasizing the critical role of newborn screening. Notably, 0.04% of cases were attributed to tap water, highlighting the importance of water source management. Additionally, incomplete hand sanitization by healthcare staff contributed to 0.04% of cases, but targeted education efforts successfully addressed this issue. These findings underscore the diverse causes of sepsis and the importance of screening and hygiene practices in reducing neonatal sepsis rates. This study has also brought attention to the potential impact of COVID-19 on the occurrence of HAIs. One possible explanation for this could be the decreased hospital footfall during COVID. The recent COVID-19 pandemic has raised concerns regarding adherence to IPC guidelines [39]. In this study, analyzing the trends over a 5-year period (2018–2023), it was observed that during the COVID-19 pandemic (2020–2022), there was a reduction in the rates of SSI, VAP, and CAUTI occurrence. In contrast, there was an increase in the incidence of CLABSI. These observations align with studies by [40,41] which also reported decreased rates of CAUTI, CLABSI, and SSI. However, a study by McMullen [42] in 2020 supported the findings of this study with increased CLABSI rates during the COVID-19 pandemic. CLABSI rates spiked during COVID-19 due to reduced low-risk admissions, shifting the denominator towards high-risk patients. COVID-19 patients, with critical illness and prolonged hospitalization, had increased central access needs, notably femoral lines, raising CLABSI risk. The strain on healthcare providers may have led to delayed CL removal and less emphasis on lower-risk venous access [42]. This situation highlighted the critical importance of strict adherence to hand hygiene and rigorous implementation of standard IPCs as recommended by the WHO and the CDC, resulting in a marked decline in HAIs. Active infection surveillance is internationally recognized for reducing infection incidence in hospitalized neonates [34]. A systematic review and meta-analyses revealed exemplary potential to reduce HAI rates significantly, 35%-55%) through multifaceted interventions, regardless of a country's income level [43]. The prevention of HAIs has witnessed substantial success in healthcare quality improvement, particularly in NICUs, by employing potential preventive strategies. This transformation is evident in the shift from viewing HAIs as unavoidable to recognizing them as preventable [36]. Several fundamental principles underscore the development of IPC strategies. These encompass, among others, enhancing hand hygiene practices, implementing effective housekeeping procedures, optimizing nurse-to-patient ratios, promoting the use of human milk, advocating for probiotics, embracing kangaroo mother care, and reducing the reliance on invasive medical devices and unnecessary antibiotic prophylaxis [44]. In a recent observational study conducted in a large gynecology/obstetrics tertiary care center, it was observed that, a multimodal strategy focusing on behavior change markedly enhanced and maintained hand hygiene practices, ultimately leading to the reduction in HAIs [45]. Various strategies have been employed to reduce HAIs. One effective approach is the care bundle, a collection of evidence-based practices consistently shown to significantly lower HAIs [5,46]. Embracing this care bundle strategy is crucial, especially in resource constrained NICUs in countries like India. Studies, both in developed and some developing nations, have demonstrated the efficacy of the CLABSI bundle in substantially reducing CLABSI rates, with reductions ranging from 59% to as high as 71% from the baseline [5]. Similarly, the recently proposed ZAP-VAP prevention bundle has shown significant effectiveness in reducing VAP rates among neonates in the NICU. The implementation of the ZAP-VAP bundle resulted in a notable reduction in VAP rates, from 8.5 to 2.5 cases per 1000 ventilator days post-intervention [47]. In the context of explaining the lower reported rates of SSI, VAP, CLABSI, and CAUTI in this study, it is crucial to outline the proactive measures implemented by Cloud nine hospitals to address infections. For CLABSI, early removal of central access lines in favor of peripheral lines, strict adherence to care bundles, rational antibiotic use, and continuous education for HCWs were key strategies. In managing CAUTI, the careful assessment of catheter necessity, timely removal (within 8-12 h for catheterized clients), and strict adherence to insertion and maintenance bundles were emphasized. VAP rates were curtailed through early ventilator weaning, preferring non-invasive ventilation methods when feasible. SSI prevention involved meticulous practices such as hair removal through clipping, pre-operative showers with 4% chlorhexidine gluconate (CHG), timely administration of prophylactic antibiotics, post-operative normothermia maintenance, wound care education, and rigorous cleaning and disinfection of operating theaters and equipment. Hand hygiene practices were enhanced through regular training, auditing based on the WHO five moments of hand hygiene, feedback mechanisms, and the display of awareness posters. Housekeeping services were meticulous, ensuring effective cleaning of surfaces and equipment during and after each customer's stay. Supervised cleaning and disinfection occurred at critical junctures in the operating theater, and Central Sterile Services Department (CSSD) practices ensured the thorough cleaning, disinfection, and sterilization of instruments with continuous quality monitoring. These multifaceted efforts collectively contribute to the observed lower infection rates in our study, underscoring the importance of a comprehensive and vigilant approach to HAI prevention. Despite advancements, notable knowledge gaps persist in implementing IPC measures in neonatal care, highlighting the need for further research and improvement in this critical area [7]. IPC is a critical, evidence-based approach aimed at safeguarding both patients and healthcare workers in every healthcare interaction [2]. The primary goal of IPC is to prevent harm by halting the transmission of infections and the emergence of antimicrobial resistance (AMR) across the entire health system. Alarmingly, there are significant gaps in IPC measures, especially in LMICs, underscoring the urgent need for increased attention, support, and resources to strengthen IPC programs worldwide, thereby enhancing patient safety and reducing the burden of HAIs. The 3+I Classification Framework, as highlighted by Garcia [48] effectively classifies interventions into four key mechanistic pathways: Primary prevention, Detection, Case management, and Implementation (3 + I). This framework underscores the significance of primary prevention, particularly in the context of central line catheters and mechanical ventilators. The low rates of different HAIs within CHN underscores the effectiveness of targeted interventions and specialized IPC measures. This study emphasized the crucial need for an enhanced focus on IPC within hospital care, highlighting the importance of dedicated professionals, allocated budgets, surveillance, and suitable patient-staff ratios.
CONCLUSION
Addressing HAIs is a critical imperative in healthcare, particularly in NICUs. Comprehensive epidemiological analysis and understanding of risk factors are vital in designing effective prevention strategies. Adherence to aseptic practices, IPC measures, and comprehensive care maintenance protocols are essential components of mitigating HAIs. The study conducted at CHN serves as a valuable prototype for similar HCFs, accentuating the need for ongoing research and customized strategies to successfully address and curb HAIs in this specific context.
CHN hospitals implemented proactive measures, such as timely interventions and careful practices, to reduce infections. These efforts resulted in lower infection rates, underscoring the importance of a comprehensive approach to preventing healthcare-associated infections. The insights gained from this study can guide the development and implementation of targeted approaches to enhance IPC, ultimately improving patient outcomes and the overall quality of healthcare delivery.
No Files Found
Share Your Publication :