-
Publish Your Research/Review Articles in our High Quality Journal for just USD $99*+Taxes( *T&C Apply)
Offer Ends On
Arif AF*
Corresponding Author: Arif A Faruqui, Department of Pharmacology, A 504, Rizvi Mahal, Opp. K.B. Bhabha Hospital, Waterfield Road, Bandra West 400050, India
Received: November 04, 2021 ; Revised: November 26, 2021 ; Accepted: November 29, 2021
Citation: Arif AF. (2021) Taurine in Pregnancy: A “Very Essential” Amino Acid. J Women Health Gynecol Res, 1(1): 1-10.
Copyrights: ©2021 Arif AF. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Views & Citations
Likes & Shares
Abstract
During pregnancy, the mother undergoes significant anatomical and physiological changes to nurture and accommodate the developing fetus. These changes soon begin after conception and affect every organ system of the mother’s body. The placenta plays a very crucial role in fetal growth as it is an important interface between mother and fetus regulating fetal-maternal exchange of nutrients, gases, water, ions and waste products. Amino acids represent one of the major nutrients for fetal life during pregnancy as both maternal and fetal concentrations are significantly higher. Taurine is considered as a conditionally essential amino acid for the fetus and it is believed that fetal taurine supply depends entirely on placental transfer from maternal plasma. In contrast to adults, fetal and placental tissues cannot synthesize taurine from cysteine and methionine as they lack the necessary enzymes. Compared to maternal plasma, the placental taurine concentration is many times higher suggesting it to be an essential amino acid required for normal growth and development. The current article discusses pregnancy complications like fetal growth retardation, pre-eclampsia and maternal obesity and the roles played by taurine which could be used as a preventive and treatment strategy for these complications.
Keywords: Placenta, Amino acids, Taurine, Fetal growth retardation, Pre-eclampsia, Maternal obesity
INTRODUCTION
Taurine (2-aminoethanosulphonic acid), a sulphur containing amino acid, is one of the most abundant amino acids in the human body as it is not used for protein synthesis [1]. Taurine is highly conserved and is present at a high concentration in many cell types allowing it to perform a wide variety of functions which collectively benefit cell and tissue wellbeing. Taurine differs from other amino acids in that it is a β and not an α-amino acid and it contains a sulfonate group instead of a carboxylate group [2]. The presence of a carboxylate group means that taurine has no net charge and is therefore a zwitterion. This zwitterionic nature infers water solubility and low lipophilicity to taurine that allows slow diffusion through lipophilic membranes [3].
Taurine is ubiquitous throughout the human body with the highest concentrations in bile (200 μmol/L), breast milk (337 μmol/L), retina (30-40 μmol/g) and platelets (16-24 μmol/g) [4]. High intracellular concentrations of taurine can be achieved due to the action of the taurine transporter, the lipophobic properties of taurine and the fact that taurine is not metabolized or incorporated into protein and therefore remains free in the intracellular environment [3, 5].
The main dietary sources of taurine are meat and seafood; however, in adults endogenous synthesis occurs in the liver from cysteine and methionine via the cysteine sulfinic acid pathway (Figure 1) [1].
FUNCTIONS OF TAURINE
Taurine has a unique chemical structure that implies important physiological functions such as bile acid conjugation and cholestasis prevention, antiarrhythmic/inotropic/chronotropic effects, central nervous system neuromodulation, retinal development and function, endocrine/metabolic effects and antioxidant/anti-inflammatory properties. Taurine is an essential amino acid for preterm neonates and is provided by breast milk [4].
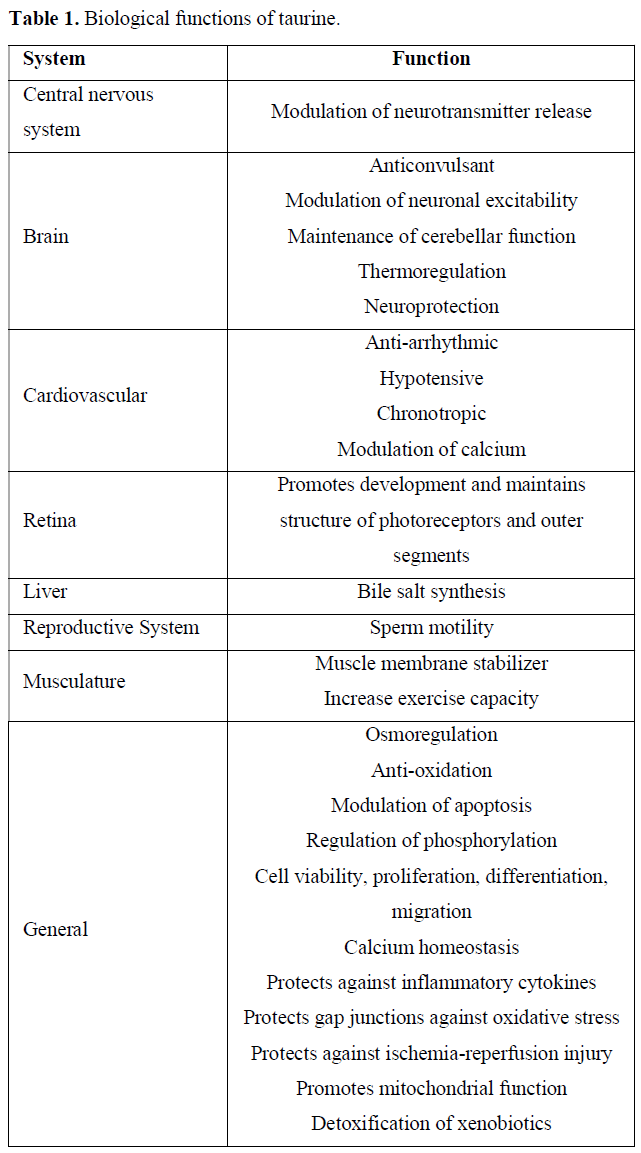
Evidences suggest taurine to be a conditionally essential amino acid in diseases associated with increased oxidative stress and inflammation, such as diabetes mellitus, obesity, metabolic syndrome and atherosclerosis. Several studies demonstrate an inverse association between plasma taurine concentrations and fasting plasma sugar as well as diabetes complications, suggesting protective role of taurine in the progression of diabetes. Taurine is a functional nutrient involved in many biological functions (Table 1) such as osmoregulation, detoxification, calcium homeostasis, neuromodulation, and cytoprotection [6].
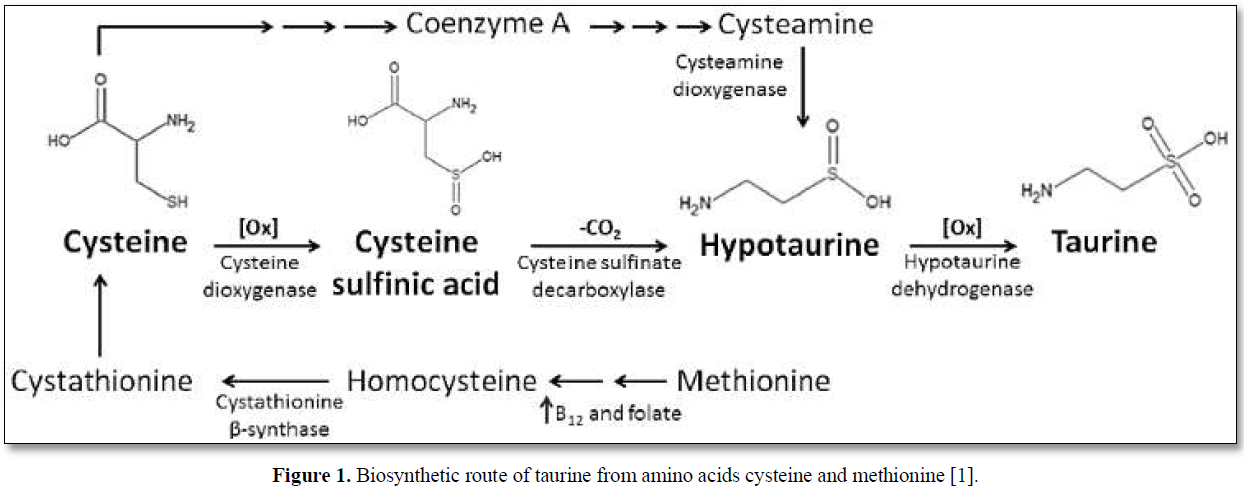
TAURINE TRANSPORT
High intracellular taurine concentration is maintained by the presence of a specific active transporter that concentrates taurine inside the cells against gradients. The taurine transporter (TauT), encoded by the solute carrier family 6-member 6 (SLC6A6) gene, is a sodium and chloride ion-dependent transporter ubiquitously expressed in mammalian tissues [1]. The transport of taurine into cells occurs by secondary active transport which involves utilizing the movement of Na+ down its electrochemical gradient to provide the energy for taurine uptake [5]. TauT transports Na+, Cl- and taurine into cells in the ratio 2:1:1 [7-9].
The concentration of taurine is 100-fold less in the plasma than in the placental tissues, suggesting that it is indeed required for modulating key cellular functions. Due to the high tissue concentration, taurine works as an osmolyte. Its cellular efflux via volume-dependent or volume-independent pathways works to osmotically balance the excessive production of metabolic by-products. Both uptake systems and efflux pathways are tightly regulated at transcriptional and post-transcriptional level, leading to an accurate control of taurine intracellular levels [1].
Pathological problems are observed when cellular taurine is depleted which is observed in as little as 48 h when the transporter is either blocked by a competitive inhibitor [10] or outright depleted [11]. The reasons for this depletion are that intracellular synthesis of taurine is limited and cells appear to be dependent on taurine uptake from blood, and the average serum concentration appears to be 20-100uM (up to 100-fold less than cells), which is reason for the active transport via TauT as taurine uptake works against a concentration gradient [11].
SYNCYTIOTROPHOBLAST FUNCTION AND TAURINE
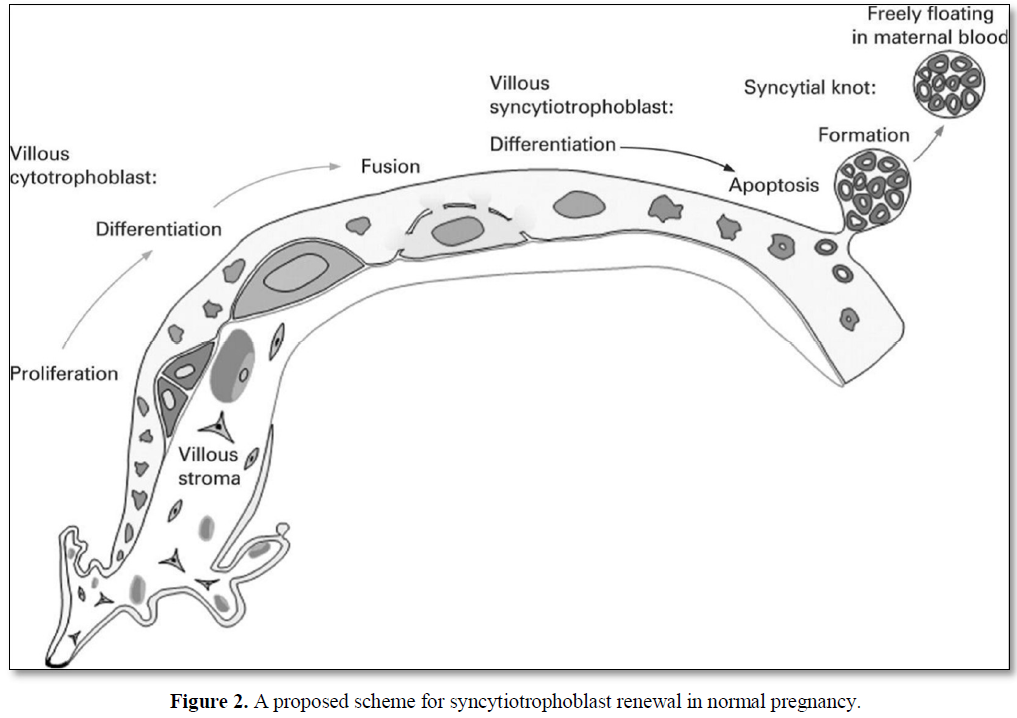
The outermost cell layer of the human placenta, the syncytiotrophoblast (STB), is a highly specialized nutrient transporting epithelium that forms a continuous multinucleated layer surrounding the villous tree. STB has an apical, maternal facing microvillous membrane (MVM) in contact with maternal blood and a fetal facing basal membrane (BM) in close proximity to the fetal capillaries. The STB is terminally differentiated and must therefore be maintained by fusion of underlying cytotrophoblasts (CTBs). CTBs are mononucleated progenitor cells which maintain the STB by undergoing proliferation and differentiation before finally fusing into the STB. To maintain the CTB population, when CTBs proliferate, one daughter cell remains as a progenitor cell whilst the other goes on to differentiate and fuse into the STB. This process of trophoblast cell turnover maintains a functional STM capable of sufficient nutrient transfer from mother to fetus (Figure 2) [12]. STB functions as a solute-transporting epithelium and endocrine/paracrine organ, delivering nutrients to fetus and producing hormones that sustain pregnancy. Consequently, maintenance of the STB is vital for a successful pregnancy [13].
Taurine is the most abundant free amino acid in human placenta (intracellular concentration ~10 mM, maternal and fetal plasma 60 and 135 mM, respectively). The high intracellular levels of taurine in the STB reflects the cytoprotective functions of taurine and also its role as an osmolyte, important for cell volume regulation, as found in other cell types [13].
Also in vitro studies have demonstrated that TauT activity in the fetal-facing BM of the STB is only 6% of that measured in the maternal-facing MVM, suggesting that the high concentration of taurine found within the STB is achieved by the uptake from maternal blood [14].
A study investigating the role of taurine in trophoblast turnover was conducted using RNA interference to deplete primary human trophoblast cells of TauT and reduce intracellular taurine content. Trophoblast differentiation was compromised in TauT-deficient cells and susceptibility of these cells to an inflammatory cytokine that is elevated in fetal growth retardation was increased, evidenced by elevated levels of apoptosis. The study data suggests an important role for taurine in trophoblast turnover and cytoprotection [13].
TAURINE IN PREGNANCY
Taurine is required for normal fetal growth and development
Taurine is conditionally essential for the growing fetus. In contrast to adults, fetal and placental tissues cannot synthesize taurine from cysteine and methionine as they lack the necessary enzymes [15]. Therefore, the fetal tissues rely entirely on the supply of taurine from the mother and the transport across the placenta. The highest concentrations of taurine are found in the fetus in the developing brain when the concentrations of other amino acids are relatively low, highlighting the importance of taurine for brain development. As development progresses the concentration of taurine in the brain falls producing concentrations in adults about a third of those found in neonates [3].
In humans, the concentration of taurine in placenta was found to be significantly lower in low-birth-weight infants compared to normally grown infants [16]. It has also been demonstrated that cord venous plasma taurine concentration is lower in fetal growth retardation compared to normally grown fetuses suggesting the involvement of taurine in this pregnancy pathology [17]. These studies demonstrate the importance of taurine for fetal growth and brain, kidney, retinal and muscle development.
The importance of taurine for neonatal development is underscored by its addition to infant formulas. Formula fed infants had higher free amino acid concentrations in both plasma and urine compared to human milk fed infants with taurine being the notable exception. The plasma and urine concentration of taurine in formula fed infants fell throughout the duration of the studies [18]. Falling urine taurine values are indicative of an attempt to conserve taurine by the kidney [19].
Children receiving >95% of their calorie intake from parenteral solutions, deficient in taurine, for extended periods had reduced plasma taurine concentrations and retinal abnormalities were corrected by the addition of taurine. Even the plasma taurine level was significantly reduced in those adults who absorbed less than 25% of their nutritional needs from their diet. These observations indicate that children, and possibly adults, receiving long-term total parenteral nutrition have a nutritional requirement for taurine [20]. The findings of these studies and the conditionally essential nature of taurine in newborn nutrition led to the addition of taurine to infant formulas from 1981 [19,21].
The three main functions attributed to taurine that might be important in maintaining STB in health and diseases are:
These are further discussed in the following sections.
Osmoregulation
The osmoregulatory role played by taurine is one of the longest recognized functions. Due to the physiochemical properties of taurine, it meets almost perfectly the characteristics for the ideal osmoregulator leading to it becoming known as a compatible osmolyte [3,5].
Following osmotic swelling taurine is lost from the cell via a volume sensitive taurine efflux pathway and the uptake of taurine via TauT is decreased [22]. As taurine is not metabolized, the activity of both TauT and the taurine efflux pathway are responsible for determining intracellular concentrations of taurine. The osmoregulatory role of taurine has been demonstrated in several non-placental tissues, including the heart, brain and renal cells [23] and in the STB [24].
Therefore, it is necessary for cells to maintain high intracellular taurine in order to facilitate maintenance of cellular hydration state. In turn, maintenance of hydration state is essential for tissue renewal processes such as cell proliferation, differentiation and apoptosis [25]. Therefore, reduction in intracellular taurine concentration may contribute to altered STB renewal involving altered proliferation and apoptosis.
Role of taurine in cell proliferation, differentiation and fusion
A number of mechanisms have been proposed for the pro-proliferative effects of taurine. It seems that taurine does not act as a mitogen itself but has a stimulatory effect once a cell has initiated proliferation [26].
It has also been noted that rather than directly affecting the DNA replication process, taurine acts by increasing the number of healthy cells and provides better conditions for the cell to enter the proliferative cycle. The study also showed that taurine increased the proportion of cells in the S phase (DNA replication stage) of the proliferation cycle, and decreased the proportion in the G0/G1 phase (resting phase) without affecting the length of the cell cycle. Taurine may also improve conditions to encourage transition between different phases of the cell cycle [27].
Taurine has been demonstrated to be important for differentiation and fusion of placenta and skeletal muscle. STB and skeletal muscle are fairly unique in that fusion to make syncytia is part of their differentiation process. Taurine supplementation has been shown to promote differentiation and fusion of mouse myoblasts in culture [28]. In addition, reduced intracellular taurine following knockdown of TauT has been shown to compromise differentiation and fusion in primary CTBs [13].
Taurine protects against ischemia-reperfusion injury and oxidative stress
Taurine is cytoprotective in a number of ways; it protects against ischemia-reperfusion injury, inflammatory cytokines and oxidative stress through its role as an antioxidant.
ROS generation is a major phase of ischemia reperfusion injury and an important determinant of the outcome following such an injury. As the rate of ROS generation is dependent on oxygen availability, during the ischemic insult the ROS generation is low, however still sufficient to cause damage. During reperfusion there is a massive influx of oxygen and consequently a significant increase in ROS production. Taurine is protective against ischemia-reperfusion injury as it regulates several events that determine the outcome of ischemia-reperfusion, including inflammation, maintenance of ion homeostasis, osmoregulation and antioxidant capacity [8].
Emerging evidence suggests the ability of taurine to prevent ROS formation may be due to its role in protecting mitochondrial function. Damaged mitochondria and defects in mitochondrial protein synthesis and electron transport chain activity can lead to a severe increase in mitochondrial oxidative stress generation. It has been demonstrated that taurine is required for the efficient translation of mitochondrial proteins and respiratory complex formation which are essential to maintain normal mitochondrial function [10,29].
Several studies have shown that taurine attenuates the effects of ischemia-reperfusion injury in rat hearts by increasing antioxidant availability, decreasing lipid peroxide accumulation and reducing apoptosis/necrosis [30-32].
In humans, taurine has been shown to have a clinical protective benefit against ischemia-reperfusion injury during coronary bypass surgery. Taurine supplementation three hours prior to surgery significantly reduced lipid peroxidation, mitochondrial cell damage and myocardial necrosis during reperfusion compared to a placebo group [33].
TAURINE DEFICIENCY IN PREGNANCY COMPLICATIONS
Fetal Growth Retardation (FGR)
A study by Norberg et al. found that TauT activity in MVM of placentas from pregnancies complicated by FGR was reduced by 34% compared with normal pregnancies [14].
In another study by Desforges [13] a 64% reduction in trophoblast TauT activity was responsible for a 66% decrease in intracellular taurine accumulation. It is therefore probable that intracellular taurine levels may be lower in the STB of placentas from FGR pregnancies. The study also demonstrated that TauT-mediated taurine transport in trophoblast cells has a role in their fusion/differentiation and multi-nucleation, but does not influence hCG secretion. This suggests that intracellular taurine has selective effects on these two well-characterized events in trophoblast differentiation, with maintenance of normal intracellular taurine being necessary for syncytialisation, but not for hormone secretion [13].
Exactly how TauT-mediated taurine transport influences trophoblast cell fusion/multi-nucleation requires investigation but two mechanisms are proposed. Intracellular taurine may be important for intracellular signaling events, which facilitate morphological differentiation. In non-placental cells, intracellular taurine modulates expression and phosphorylation of proteins involved in the mitogen-activated protein kinase (MAPK), signal transducer and activator of transcription 3 (STAT3) and protein kinase C (PKC) signaling pathways. Each of these signaling molecules are involved in cell differentiation and therefore, alterations in intracellular taurine could affect their role in this process. Alternatively, taurine could help maintain gap junction intercellular communication required for cell fusion.
In addition to an impaired ability to differentiate morphologically, study by Desforges [13] showed that TauT-deficient trophoblast cells are more susceptible to TNFα-induced apoptosis. Elevated levels of TNF-α have been reported in FGR and this has been suggested to contribute to the increase in trophoblast apoptosis associated with these pregnancy complications. However, results demonstrated that TNF-α induces significant apoptosis in cytotrophoblast cells only when their ability to accumulate intracellular taurine has been reduced through SLC6A6-specific knockdown.
In summary, reduced TauT activity in CTB cells leads to impaired morphological differentiation and an increased susceptibility to apoptotic cell death. Such alterations in utero would have implications for STB renewal, compromising the transfer of nutrients from the maternal circulation towards the fetus via other transporters present on the MVM, and also cause disruption to the endocrine function of the STB, leading to placental insufficiency with consequences for fetal growth and wellbeing. The ability of trophoblast cells to accumulate sufficient taurine intracellularly for their differentiation and survival is therefore crucial for a healthy pregnancy [13].
Pre-eclampsia
Pre-eclampsia (PE) is a serious disease affecting approximately 5% of pregnancies each year worldwide [34]. It is a multisystem disorder characterized by systemic inflammation and endothelial dysfunction in the mother. PE has serious consequences for both mother and infant and is associated with increased maternal and fetal mortality and morbidity [35].
The etiology of PE is multifactorial and incompletely understood. However, it is recognized that the placenta is the crucial stimulus for PE as the disease has developed in molar pregnancies where only the placenta is present [36]. The origin of PE lies in the maternal response to abnormal placental development and placenta derived factors. A current hypothesis for PE pathogenesis is that abnormal uterine vascular remodeling in early pregnancy results in reduced and turbulent blood flow to the placenta which establishes an environment of oxidative/nitrative stress and inflammation. This has detrimental effects on the maintenance of STB, the nutrient transporting epithelium of the human placenta, which leads to (a) necrotic/inflammatory material being deported from the placenta into maternal blood which activates the maternal endothelium to trigger PE and (b) compromised placental nutrient and endocrine function that can compromise fetal growth [37].
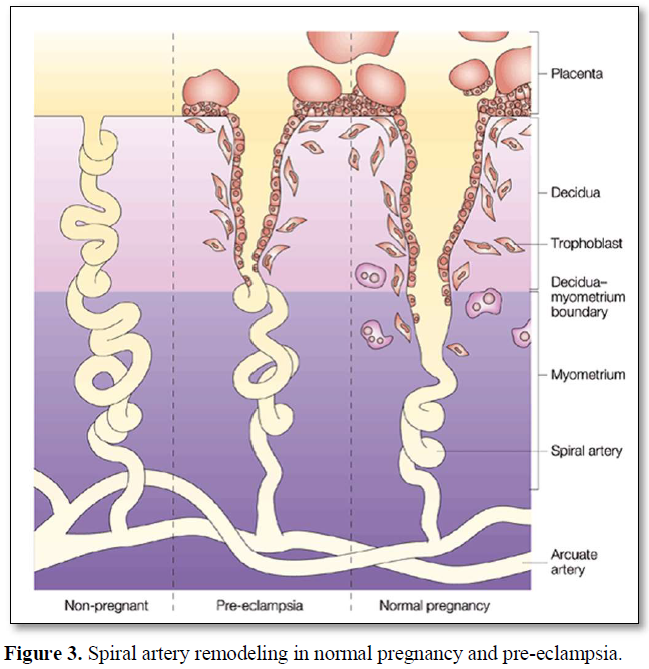
In normal pregnancy spiral arteries in the decidua and top third of the myometrium are remodeled by invading trophoblasts, producing dilated low resistance vessels. In pre-eclampsia this remodeling is limited to the decidual region which leads to reduced placental perfusion as depicted in (Figure 3) [38].
In addition to avoiding the deportation of pro-inflammatory material, STB integrity is of the utmost importance for the efficient transfer of nutrients between mother and fetus. Fetal growth is directly related to STB volume and in PE altered STB cellular renewal from proliferation through to apoptosis results in altered STB volume and thickness. Therefore, altered renewal of STB leading to compromised nutrient transfer may underlie FGR in PE [39].
Bearing in mind the functions of taurine in regulating proliferation, differentiation, fusion and apoptosis, and in offering cytoprotection it is plausible that a reduction in intracellular taurine consequent on reduced STB TauT activity in pregnancy complications could compromise STB renewal and function. Therefore, it is proposed that intracellular taurine depletion in vivo, as a consequence of reduced TauT activity, could contribute to the abnormal STB renewal that is a key feature of PE.
Maternal Obesity
There are several factors that increase the risk of a woman developing PE and of this maternal obesity is a major contributor. The risk of PE increases progressively with BMI; women with a BMI>30 in early pregnancy are 3 times more likely, and with a BMI>40 are 4 times more likely, to develop PE than women of ideal weight [40-42]. This is worrying as the rate of obesity, in particular morbid obesity, is increasing worldwide.
As the incidence of maternal obesity is rising it is expected that the incidence of PE will increase in parallel. In common with PE, dysregulation of STB turnover is evident in maternal obesity [43] and both conditions are associated with increased placental oxidative/nitrative stress [44,45]. In addition, laboratory studies have shown that the uptake of taurine into the STB is significantly lower in obese women compared to women of ideal weight, indicating another potential abnormality common to obesity and PE [46].
Taurine plays a very important role in reducing fat deposition by modulating cellular pathways for lipid accumulation and stimulating mobility. Taurine was effective in treating fatty liver of children with simple obesity regardless of the success/failure of weight control in a study. Synthetic activity and concentration of taurine in adipose tissues and plasma have been shown to decrease in humans and animals during the development of obesity, suggesting a strong relationship between taurine deficiency and obesity [47].
Possible mechanisms responsible for the anti-obesity effects of taurine are shown in (Figure 4) [48].
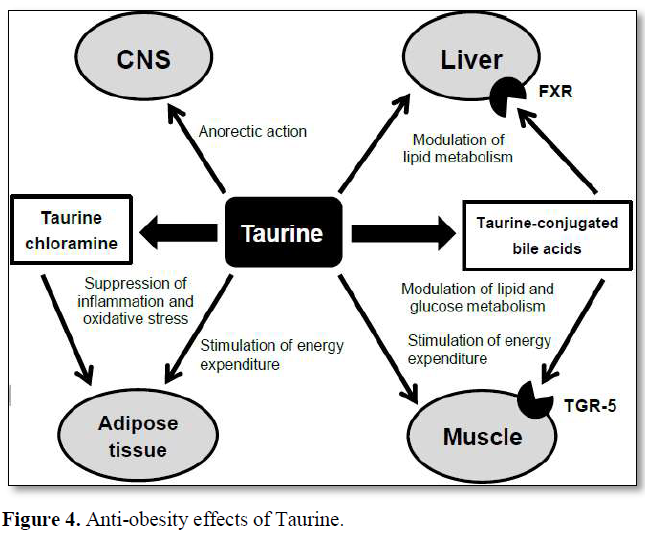
Taurine reduces tissue and plasma cholesterol levels by stimulating bile acid conjugated bile acids. Taurine-conjugated bile acids are also the ligand for the G protein-coupled receptor TGR-5. Activation of the TGR-5 leads to enhanced energy production, increased glycemic control and anti-inflammatory effects. Taurine stimulates energy production in adipocytes. In inflamed adipocytes, taurine is converted to taurine chloramine by inflammatory cells, including macrophages and exhibits anti-inflammatory and antioxidative functions. In the CNS, taurine exhibits an anorectic effect in the hypothalamus by enhancing the insulin signaling pathway. The combination of these direct and indirect actions of taurine results in the anti-obesity effects [48].
Independent of increasing the risk of PE, there are serious complications associated with obesity synthesis from cholesterol and eliminating it from the body. Taurine increases hepatic mitochondrial β-oxidation of fatty acids and thereby, ameliorates the accumulation of triglycerides in plasma and tissues. In addition, taurine modulates lipid metabolism through the activation of the nuclear farnesoid X receptor (FXR) after converting to taurine in pregnancy for both mother and fetus. Detrimental effects include increased likelihood of large for gestational age (LGA) births, stillbirth with FGR, premature delivery, miscarriage, congenital abnormalities and neonatal death [49-51]. As there is a wealth of evidence that disorders of fetal growth are associated with cardiovascular and metabolic disease in later life, obesity in pregnancy severely impacts on the health of the next generation [52,53].
Taurine role in pregnancy complications
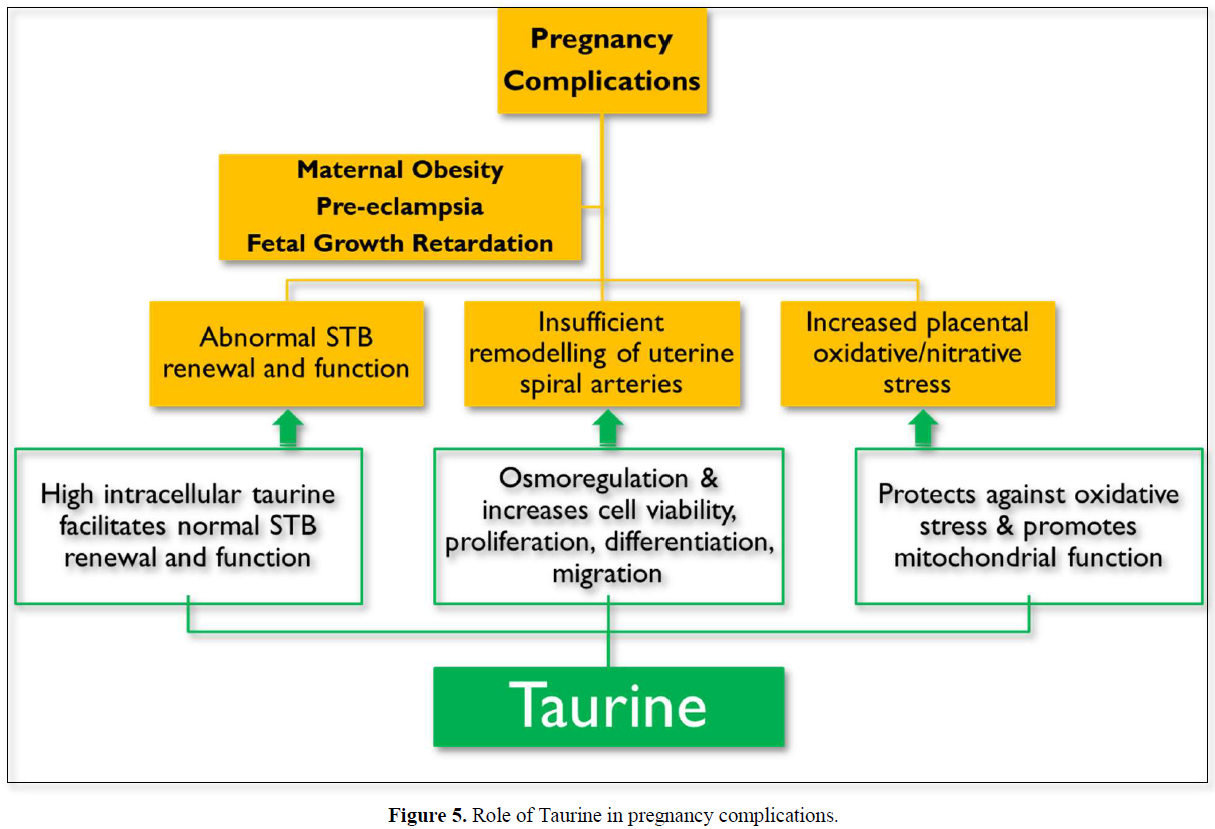
PE, FGR and maternal obesity are associated with abnormal STB renewal and function, insufficient remodeling of uterine spiral arteries and an increased placental oxidative/nitrative stress. Taurine supplementation could ameliorate all these pregnancy complications due to its diverse actions summarized in (Figure 5).
TAURINE DAILY INTAKE
Taurine occurs naturally in food [54]. The mean daily intake of taurine from the diet has been estimated to vary between 40 and 400 mg/day [55]. Since taurine is synthesized endogenously (the average daily synthesis is 50-125 mg), occurs naturally in foods and any excess of taurine is rapidly eliminated by the kidneys, no safety concern was observed for the intake value of 21 mg/kg body weight per day in the risk assessment of taurine by the Norwegian Scientific Committee for Food Safety [56].
SUMMARY
PE and maternal obesity are serious complications of pregnancy, associated with fetal growth retardation and abnormal renewal of STB, and maternal obesity is a major risk factor for PE. Reduced TauT activity could contribute to dysregulated renewal of STB and FGR that are common to PE and maternal obesity. Taurine addresses all these complications with different biological actions and could therefore be used as a preventive and therapeutic strategy for their management.
ACKNOWLEDGEMENT
Author acknowledges the contribution of Mr. Rafique Sheikh, M. Pharm (Pharmacology) for his contribution in doing literature search & helping with the preparation of manuscript.
FINANCIAL DISCLOSURE OR FUNDING
Nil.
CONFLICT OF INTEREST
Nil.
AUTHOR CONTRIBUTIONS
Prepared and reviewed the review article.
REFERENCES
No Files Found
Share Your Publication :