-
Publish Your Research/Review Articles in our High Quality Journal for just USD $99*+Taxes( *T&C Apply)
Offer Ends On
Sonia Mor*, Vikas Dhikav, Varisha Anjum and Mojahidul Islam
Corresponding Author: Sonia Mor, Ph.D., Clinical Research, Lingayas Vidyapeeth, Faridabad and Indraprastha Apollo Hospitals, New Delhi, India
Received: January 07, 2022 ; Revised: February 11, 2022 ; Accepted: February 14, 2022
Citation: Mor S, Dhikav V, Anjum V & Islam M. (2022) Review on Status Quo of COVID-19 Treatments. J Stem Cell Ther Res, 1(1): 1-7.
Copyrights: ©2022 Mor S, Dhikav V, Anjum V & Islam M. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Views & Citations
Likes & Shares
Abstract
Complementary and Alternative medicines (CAM) or other natural products on boosting one’s immunity against COVID-19 have potential to improve or supplement existing standard of care available to prevent or treat COVID-19. Pandemic has brought the world’s focus on health back again. COVID-19 has shown its high virulence in an immune-compromised population whereas it is generally mild in otherwise healthy people. It is hence, important to enhance the body’s natural defense system using a healthy diet, and an active lifestyle to maintaining optimum health. A lot of organized scientific studies on therapies for COVID-19, both locally and internationally, are being quickly produced. Therefore, credible evidence supported by appropriate research is essential to ensuring public health in dealing with the current COVID-19 pandemic and improving public awareness on the undue promotion of fake medicine. Oleander teas, essential oils, siliver products have been tried without success in USA. In India, a sizable number of people affected with n-Coronavirus infection have tried Ayurvedic Kadha, a recommended formulation with subjective health benefits. Curcumin, a polyphenolic compound extracted from spice, used commonly in India, can potentially enhance the antibody response even if it takes at low doses. Curcumin is used as Golden Milk (Haldi milk) in India as a popular immunity boosting remedy against COVID-19 infection. Similarly, Vitamin D has been suggested to be one of the CAM potentially useful in boosting immunity. Much in the same way, limited evidence suggests that steam inhalation, saline gargles, nasal irrigation, and yoga could be useful as well.
Experts have also been suggesting taking a holistic approach to general health focusing on immunity focusing on prevention before cure. Current review focuses on the role of Natural products and approaches against COVID-19.
Keywords: Complementary and alternative system of medicine, Natural products, COVID, Corona virus
Abbreviations: COVID-19: Corona Virus Disease-2019; AIIMS: All India Institute of Medical Sciences; CAM: Complementary and Alternative Systems of Medicine; HBA1C: Glycated Hemoglobin (A1c) Control; GLR: Glycyrrhizic Acid; AIST: National Institute of Advanced Industrial Science and Technology; HBOT: Hyperbaric Oxygen Therapy; EMA: European Medicines Agency; WHO: World Health Organization; USFDA: United States Food and Drug Administration; DCGI: Drugs Controller General of India; AIDS: Acquired Immune Deficiency Syndrome
INTRODUCTION TO COMPLEMENTARY AND ALTERNATIVE SYSTEM OF MEDICINE (CAM) & COVID-19
Complementary and Alternative system of Medicine (CAM) are the conventional medicines which are not fully integrated into the dominant health-care system for medical products. It refers to “the use of other sorts of drugs or therapies other than part of standard medical care, may also be called biomedicine or allopathic [1,2]. which includes a wide variety of botanicals and nutritional products, such as dietary supplements, herbal supplements, and vitamins as well as yoga other than the use of allopathic treatments, alone or along with in combination with conventional or allopathic treatments or in lieu of conventional therapies [3]. They are considered to be safe because they are present in, or produced by, nature. However, that may not be true in all cases and needs proper research before letting them come in therapeutic use just like in case with allopathic medicines.
About half the general population in developed countries uses CAM.As long as alternative treatments are used alongside conventional treatments, the majority of medical doctors find most forms of complementary medicine acceptable [4,5]. Patients with conditions like AIDS, allergy and auto-immunity disorders tends to use or seek their own solutions to sustain, restore or even boost their immune competence, live more comfortably and longer. In a study, the prevalence of CAM use was relatively high (32.9%) among HIV patients [6]. Complementary and alternative medicine use is also high among cardiac patients (56. 2%) [7]. Possibility of use of such a system of medicines increases where there are no treatments available for particular conditions like in case of COVID-19 or in case of therapeutic failures. Social and clinical contexts promise of enhanced immunity are pursued through popular sorts of practices known as complementary and alternative medicine and the evidence that supports these [8,5]. Many such examples exist e.g., Curcumin (used as Golden milk), vitamin C, vitamin D can boost immunity by their anti-oxidant mechanisms which can boost natural defense against viral attacks.
Use of CAM is believed to have health benefits [9]. Plants like Echinacea, Cinchona, Curcuma longa, and Curcuma xanthorrhiza, are being explored as potential treatment for COVID-19. A recent review also suggested herbal remedies and drug repositioning to achieve the common goal of finding a safe and effective treatment for COVID-19 at the earlies [10].
Another study suggested that long-term follow-up studies of recovered patients are crucial in determining the effects of medications or CAM products on organ functions due to disease or interventions [11].
THE ETIOLOGY, SYMPTOMS AND PATHOPHYSIOLOGY OF COVID-19
COVID-19 situation is very contagious and emerging situation worldwide as it is a new addition to virus family. The virus first case appeared in the city of Wuhan, China, on December 31, 2019, and soon it has quickly spread globally through human-to-human transmission creating emerging respiratory disease outbreaks leading to life threatening situation and global health crisis [12]. The outbreak was declared as a global health emergency on January 30, 2020 affecting more than 210 countries worldwide in a quick span of time.
The COVID-19 infection may present with mild to moderate or severe clinical manifestations, causing severe pneumonia, as well as extra pulmonary manifestations and systemic complications such as sepsis, and septic shock. As of May 23, 2020, it has infected around 5.3 million people globally with 340,000 deaths [13].
COVID-19 is a potentially preventable disease [14]. Coronavirus can be potentially controlled using adequate nutrients; for instance, treating influenza with very large amounts of vitamin C has been practiced for decades. The common cold, SARS-CoV-1, and SARS-CoV-2 fall under the same coronavirus family; hence, are regarded as the same viral type [14]. CoVs is enveloped, positive-stranded RNA viruses with nucleocapsid.
People with co-morbidities are many fold times more likely to die of COVID-19 as is shown by a study performed by the US Centers for Disease Control and Prevention (CDC) stating that cardiovascular disease (32%), diabetes (30%) and lung disease (18%) are the most common chronic diseases among Covid-19 patients [15]. One in five (19.5%) people with underlying diseases died compared to 1.6% of those who were otherwise healthy, among 1.3 million cases in total [16].
Progressive respiratory failure occurs in severe patients and is initiated by the receptor-binding domain attachment of the virus to the receptor on the respiratory tract, known as the angiotensin-converting enzyme-2 (ACE2) receptor. Humans have many ACE2 receptors in their respiratory tracts, which increase their susceptibility to COVID-19. Subsequent inflammatory processes and the release of numerous proinflammatory cytokines that is responsible for the clinical appearance of inflammation. Some of these proinflammatory cytokines, including IL-2, IL-7, IL-10, G-CSF, TNF-α etc., are highly elevated in the blood of severely ill COVID-19 patients. Thus, there may be an association between this elevated level of cytokines and the severity of a patient’s manifestations.
ALLOPATHIC TREATMENT AND CLINICAL TRIALS OF COVID-19
Currently, scientists race to find a cure for the corona virus disease, the numbers of COVID-19 positives are severe, high fatal, with exponential increase. No effective therapeutic drug/vaccine has been announced yet. Though with very limited options, ‘off - label use of drugs’ and “repurposing of the existing drugs” are taken in an exploratory phase in order to find one standard treatment for the fatal condition. The available treatment options including the use of Remdesivir, Hydroxychloroquine, Lopinavir, Ritonavir, APN01 or Favilavir are being tested in clinical trials across the globe.
In a retrospective study [17], data demonstrated that Hydroxychloroquine treatments are highly effective in reducing the fatality of critically ill patients of COVID-19. Also, it has been seen in studies that hydroxychloroquine reduces the chances of contracting COVID 19 infections. India is the world’s biggest producer of hydroxychloroquine, which is approved for use as a prophylaxis to be given to asymptomatic health workers.
Another drug, Remdesivir, an antiviral drug that has also proven to be “superior” and most beneficial for hospitalized patients who severely required supplemental oxygen supply [18]. In India, authorized off-label use of treatments like use of tocilizumab and convalescent plasma therapy on specific groups of patients is also in practice [19].
APPROVED TREATMENTS UNDERGOING HUMAN TRIALS
In a recent review, it has been analyzed that a combination of antiviral drugs with hydroxyl-chloroquine and azithromycin may be the best option to treat the patients [20]. A combination of antiviral drugs with hydroxyl-chloroquine and azithromycin (with the consultation of a medical practitioner) may be the best option to treat the patients, depending on the patient's conditions and symptoms. However, Unani therapy may be useful along with allopathic treatment [20].
The World Health Organization (WHO), trial results announced showed that mortality rate was reduced with use of dexamethasone [21,22]. According to the preliminary findings used since the 1960s to reduce inflammation in diseases such as arthritis, cut death rates by around a third among the most severely ill COVID-19 patients admitted to hospital.
In India, another Drug candidate is approved, the use of Biocon’s biologic drug Itolizumab for the treatment of moderate to severe novel coronavirus patients [23].
Nocturnal oxygen therapy in the early stages may be helpful in preventing disease progression by inhibiting the rapid replication of the virus and improving the body’s antiviral ability [24]. Another therapy is proved to be beneficial and has shown improvement in small number of patients with HBOT (Hyperbaric oxygen therapy), potentially prevented the need for mechanical ventilation [25].
VACCINE DEVELOPMENT
To meet society's need for safe and efficacious vaccines, the clinical vaccine development and clinical evaluation of a vaccine process takes 10-15 years and requires a budget of about 1 billion US dollars [26]. Vaccine development consists of following steps e.g., exploratory, preclinical and clinical stages [27]. A human trial is the last stage of research in the development of a vaccine [28]. Regulatory agencies worldwide divide this development process into preclinical (in vitro and in vivo testing in animals) and clinical (clinical trials in human subjects) stages [29].
To plan the clinical development path of a novel vaccine candidate, guidelines have been issued by the European Medicines Agency (EMA), the World Health Organization (WHO), and the United States Food and Drug Administration (USFDA) [29].
The University of Oxford and AstraZeneca Plc.’s experimental vaccine, ChAdOx1 nCoV-19 vaccine, a weakened version of a common cold virus (adenovirus) that causes infections in chimpanzees; is the first to enter the final stages of clinical trials [30]. ChAdOx1 nCoV-19 showed an acceptable safety profile, and homologous boosting increased antibody responses, together with the induction of both humoral and cellular immune responses, support large-scale evaluation of this candidate vaccine in an ongoing phase 3 programme to look for the assessment of safety, immunogenicity and efficacy profiles of ChAdOx1 nCoV-19 against symptomatic PCR-positive COVID-19 disease in randomized controlled clinical trials in humans [30]. ChAdOx1 nCoV-19 appears to be better tolerated in older adults than in younger adults and has similar immunogenicity across all age groups after a boost dose. Further assessment of the efficacy of this vaccine is warranted in all age groups and individuals with comorbidities [31].
There are two vaccine candidates in India that are currently in human trials. One of these vaccines is a traditional formulation called Covaxin, developed by the Hyderabad-based Bharat Biotech in collaboration with the Indian Council of Medical Research [32]. The vaccine has undergone successful trial in animal models e.g., mice, rabbit and guinea pigs and following good immunogenicity of the inactivated vaccine, Phase 1/2 clinical trial in several institutions in India has already started [28].
The other candidate, developed by the Ahmedabad-based private pharma Zydus Cadila., vaccine is based on a radical technology, called ZyCoV-D, Human trials began in mid-July for both the vaccines. The Phase I trial of vaccine was conducted on healthy volunteers and was found safe in all the participants [33].
OPTIONS OF COMPLEMENTARY AND ALTERNATIVE MEDICINES
In the present situation, a very limited number of effective allopathic medicines against COVID-19 are there in use. A total dependence on discovery of new allopathic molecule or vaccine could be challenging and time taking [34]. The current recommendations include infection prevention as well as control measures and supportive treatment of COVID-19 complications [35]. Several drugs have been tried, including antimalarials, antiviral agents, immunomodulators and plasma-neutralizing antibody transfusion [36]. There is an urgent need to develop targeted therapies. Various therapeutic agents quickly taken into clinical trials are largely based on existing drugs with non-specific antiviral activities or compounds pharmacologically speculated to be effective in enhancing the overall clinical outcome of COVID-19 patients [37]. Clinical trials assessing antivirals, chloroquine, hydroxychloroquine, glucocorticoids, convalescent plasma transfusion against COVID-19 [38].
The herbal formulations as alternative medicine may contribute to the eradication of complicated viral infection significantly. Various medicinal plants, those are Sambucus nigra, Caesalpinia pulcherrima, and Hypericum connatum, holding promising specific antiviral activities scientifically proven through studies on experimental animal models [39].
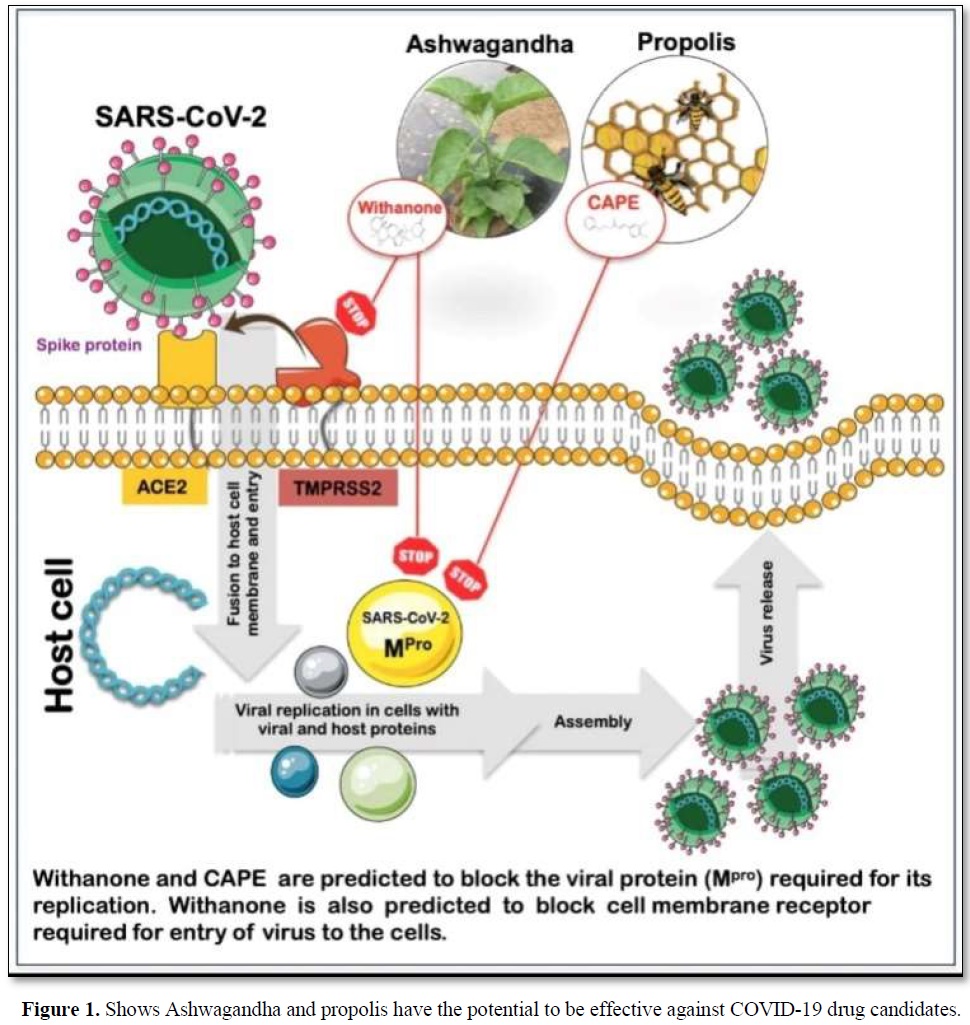
In a collaborative study [40], IIT Delhi in collaboration with AIST (National Institute of Advanced Industrial Science and Technology), an institute in Japan discovered that those natural compounds from Ashwagandha and propolis have the potential to be effective against COVID -19 drug candidates (Figure 1).
In another telephonic survey performed to assess the use of Complementary and Alternative Medicine (CAM) and home remedies by COVID-19 Patients, results showed 25.8% people used CAM products and home remedies during the treatment and even afterward, many of them consumed more than one CAM products or home remedies with no reported acute or severe adverse effects with these products [11].
More than 80% of the world's population uses Complementary and Alternative Medicines (CAMs) [41].
In another review, stated that the natural inhibitors against the SARS-CoV enzymes, such as the nsP13 helicase and 3CL protease, have been identified and include myricetin, scutellarein, and phenolic compounds from Isatis indigotica etc. [42].
Foods and herbs could be used as dietary or complementary therapy to prevent infection and strengthen immunity, as antiviral agents for masks, as disinfectants to curb aerosol transmission, or as sanitizing agents to disinfect surfaces [42].
Coumarin is one such natural compound that is a potential drug candidate showing antiviral effect, its inhibitory role against infection of various viruses such as HIV, Influenza etc. [43]. Similarly, there are proposals about the use of liquorice roots (Glyceria Glabra) chewing could be useful in prevention and treatment of COVID-19 infection.
Anti- COVID-19 Ayurvedic drug, Coronil having about than 100 compounds was announced recently in India. However, the Union Ministry confirmed that this formulation as immunity booster and not as a medicinal cure for COVID-19 [44,45].
Another homoeopathic drug [46], Arsenicum album 30, is also recommended by the Ministry of AYUSH listed the drug among “preventive and prophylactic simple remedies” against Covid-19.
Ayurveda is believed to have enough potential and possibilities to be employed both for the prevention and an adjunct treatment option for COVID-19 [47]. A case report was published, first known case of a COVID-19 positive patient treated entirely with Ayurveda medicines alone, within a short period [48].
Also, Yoga is being consistently practiced regularly and suggested by many health experts and yoga practitioners to improve immunity in order to fight the dreadful condition, COVID-19 [49-51].
Another proposed anti-microbial medicinal formula has been proved as an Ayurvedic antibiotic, called “FIFATROL”, is studied by All India Institute of Medical Sciences (AIIMS), New Delhi, India. It is prepared from 13 herbs and helpful in boosting immunity [52]. In overseas countries like US, Europe & Russia, where the pandemic has taken over badly, the demand for curcumin-rich turmeric, Ashwagandha, Amla (amalaki), Giloy Juice, chyawanprash etc. has increased greatly. In many countries of Europe, doctors have been recommending Ayurvedic medicine to patients along with allopathic medicines. Glycyrrhizin, glycyrrhizic acid (GLR) an active component of liquorice roots, also a good candidate to be tested against the SARS-CoV-2 corona virus, alone and in combination with other drugs can also be further considered and rapidly evaluated for the treatment of patients with COVID-19 [53,54].
It is believed that “Yoga” can be performed regularly to de-stress the mind and body and can strengthen the body and lungs leading to boosting one’s immunity with reference to fighting against COVID-19. Also, adaptive Clinical trials are in practice to develop a new line of drugs and vaccine development has been initiated world-wide.
Therefore, Yoga and Ayurveda are potentially capable of giving health, immunity for COVID-19.
It is worth to further assess the benefits of CAMs, especially vitamins, for prevention and treatment of infectious diseases in general and COVID-19 in particular in the early stage of infection [55,56].
CONCLUSION
Though above mentioned potential complementary therapies and alternatives claims to be effective and look promising in present emergent situation but long-term safety and efficacy data needs to be scientific base for evidence that drugs of Complimanry & Alternative System of Medicine (CAM) work against Covid-19. Randomized controlled trials should be encouraged to gather more information for the treatment of novel coronavirus. Counting on historic data, leads about use of Yoga, steam and haldi/salines gargles/nasal irrigation, and use of Kadha shows promising results in mild to moderate COVID-19 cases but for those who are hospitalized in need of oxygen or advanced treatments, incorporation of such therapies along with should be encouraged and monitored properly to have a conclusive opinion of their use.
CONFLICT OF INTEREST
No financial contributions and any potential conflict of interest.
ACKNOWLEDGEMENTS
All individuals listed as authors have contributed substantially to the design, performance, analysis, or reporting of the work and required to indicate their specific contribution. Anyone (individual/company/institution) who has substantially contributed to the study for important intellectual content, or who was involved in the article’s drafting the manuscript or revising must also be acknowledged.
SUPPLEMENTARY MATERIAL
Supportive/Supplementary material intended for publication are numbered and referred to in the manuscript but should not be a part of the submitted paper.
REFERENCES
No Files Found
Share Your Publication :