-
Publish Your Research/Review Articles in our High Quality Journal for just USD $99*+Taxes( *T&C Apply)
Offer Ends On
Aakanksha Sinha*, Ritika Shrivastava and SJ Daharwal
Corresponding Author: Aakanksha Sinha, University Institute of Pharmacy, Pt. Ravishanker Shukla University, Raipur (C.G.) 492001, India.
Received: March 19, 2025 ; Revised: March 27, 2025 ; Accepted: March 30, 2025 ; Available Online: April 09, 2025
Citation: Sinha A, Shrivastava R & Daharwal SJ. (2025) Current Trends in Analytical and Bioanalytical Methods for Etodolac: A Review. J Pharm Sci Drug Discov, 4(1): 1-6.
Copyrights: ©2025 Sinha A, Shrivastava R & Daharwal SJ. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Views & Citations
Likes & Shares
Etodolac belongs to a class of nonsteroidal anti-inflammatory drug (NSAID). Its therapeutic effect is due to inhibition of prostaglandin synthesis. The development of analytical techniques and the many approaches now in use for etodolac estimate, whether in bulk or pharmaceutical dose form, are the main focus of the current study. Analytical procedures are crucial for determining compositions because they allow us to obtain both qualitative and quantitative data using state-of-the-art analytical equipment. Chromatographic, electrochemical, spectroscopic, and other methods can be used to analyze it. These methods aid in comprehending important process factors and reducing the detrimental influence they have on accuracy and precision. The development of analytical methods is required to meet regulatory requirements and maintain high standards for the quality of commercial products. In response to the reference, regulatory bodies in a number of nations have developed guidelines and procedures for approving, authenticating, and registering.
Keywords: Etodolac, UV-Visible Spectrophotometer, HPLC, HPTLC, Bioanalytical method
INTRODUCTION
Etodolac is an NSAID that belongs to the pyranocarboxylic acid class. It is an organic heterotricyclic molecule and a monocarboxylic acid. It inhibits prostaglandin synthesis and cyclooxygenase 2 inhibitor. It is used to treat the symptoms of osteoarthritis and rheumatoid arthritis. It functions as an antipyretic, non-narcotic analgesic, and non-steroidal anti-inflammatory medication. It comes in extended release, immediate release tablets and capsules. The S-form of etodolac enantiomers is biologically active [1].
PHYSICOCHEMICAL PROPERTIES
Etodolac is white, crystalline substance. It is soluble in alcohols, chloroform, dimethyl sulfoxide, and aqueous polyethylene glycol but insoluble in water. Its molecular weight is 287.35 g/mol. Its melting point is 145-148 ºC. Its pKa is 4.65 [2].

PHARMACOKINETIC
The systemic bioavailability of etodolac in tablet or capsule form is at least 80%, according to mass balance tests. More than 99 percent is protein bound, mostly to albumin. Its half-life is six to eight hours. The liver metabolizes etodolac substantially. The main excretion pathway for etodolac and its metabolites is the kidneys (72%) [3].
PHARMACODYNAMIC
Etodolac has analgesic and antipyretic qualities and is an anti-inflammatory drug. It is used to manage acute pain and treat rheumatoid arthritis and osteoarthritis. Etodolac works therapeutically by preventing the production of prostaglandins that cause fever, discomfort, edema, and inflammation. As a racemate, etodolac is administered. It has been demonstrated that the R-form is inactive and the S-form is active, similar to other NSAIDs [4].
MECHANISM OF ACTION
Etodolac's anti-inflammatory effects, like those of other NSAIDs, are caused by suppression of the cyclooxygenase (COX) enzyme. As a result, fewer peripheral prostaglandins that mediate inflammation are synthesized. Etodolac blocks the entry of arachidonic acid, the substrate of the COX enzyme, by attaching itself to the top part of the active site. Etodolac is now recognized to be more selective for COX-2 than COX-1, despite the fact that it was once believed to be a non-selective COX inhibitor. By acting centrally on the hypothalamus, antipyresis can cause peripheral dilatation, elevated cutaneous blood flow, and consequent heat loss [5].
NEED OF ANALYTICAL METHOD DEVELOPMENT
Analytical technique development culminates in official test procedures. Quality control labs therefore employed these methods to analyze the efficacy, safety, purity, performance, and identification of pharmaceutical products. For regulatory agencies, production-related analytical methods are extremely important. To get the medicine approved by regulatory bodies, the applicant must show that they have complete control over the drug development process using recognized analytical methodologies [15]. Recent analytical guideline documents produced by the ICH include stability testing (Q1), analytical technique validation (Q2), contaminants in drug substances and products (Q3), and specifications for novel drug substances and products (Q6) [6].
ANALYTICAL METHOD DEVELOPMENT BY UV-VISIBLE SPECTROSCOPY
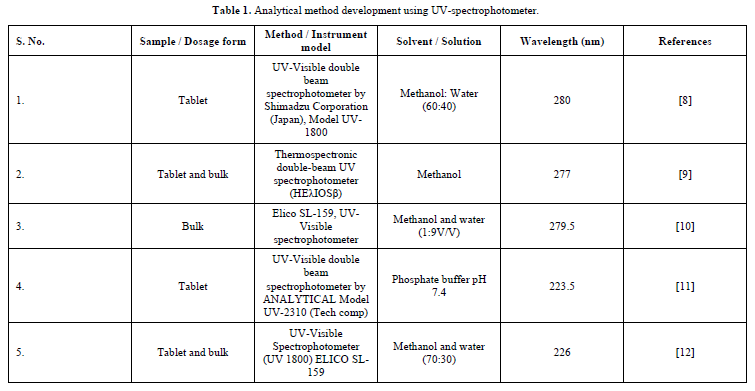
Under UV-visible spectroscopy is the study of interactions between matter and electromagnetic radiation in the UV-visible spectrum. The ultraviolet's (UV) wavelength range is 200-400 nm. The basis for this is the Beer-Lambert law, which states that the absorbance of a solution and the length of its journey are directly proportional. For a given path length, it can therefore be used to determine the absorber's concentration in a solution. The rate at which absorbance changes with concentration must be understood [7] (Table 1).
ANALYTICAL METHOD DEVELOPMENT BY HPLC
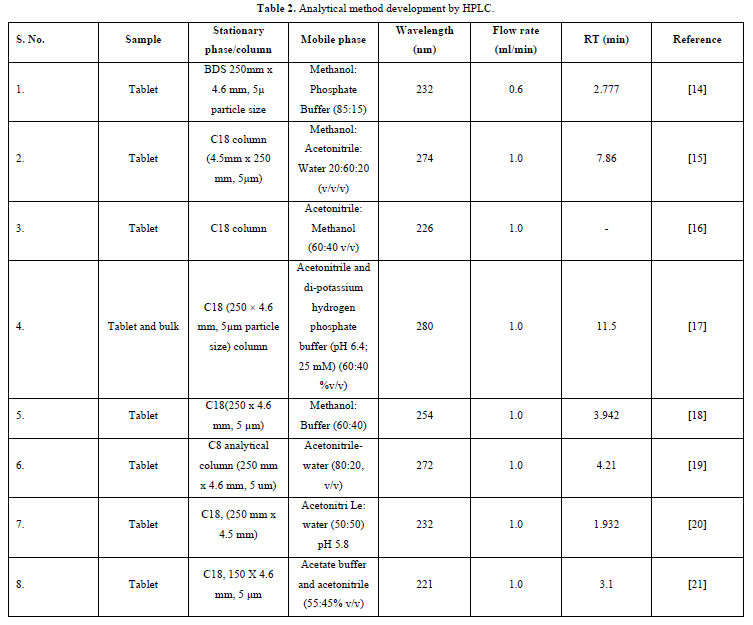
Among the most widely used separation techniques, high performance liquid chromatography (HPLC) is one of the most well-established analytical processes. It has been used in labs worldwide for more than 40 years for evaluations of food and the environment, clinical chemistry, pharmaceutical sciences, synthetic chemistry, etc. The stationary phase in this method could be either a liquid or a solid phase. HPLC can be used to separate a combination's constituent parts by employing a liquid mobile phase. When the stationary phase is housed within a column and the liquid mobile phase is mechanically pumped through the column, the process is known as "high-performance liquid chromatography" (HPLC). The column is a vital component in HPLC systems. A good silica and bonding process will result in a reproducible and symmetrical peak, which is necessary for accurate certification. Commonly used RP columns include Cyno (USP L18), Phenyl (USP L11), C18 (USP L1), and C8 (USPPL8) [13] (Table 2).
ANALYTICAL METHOD DEVELOPMENT BY HPTLC
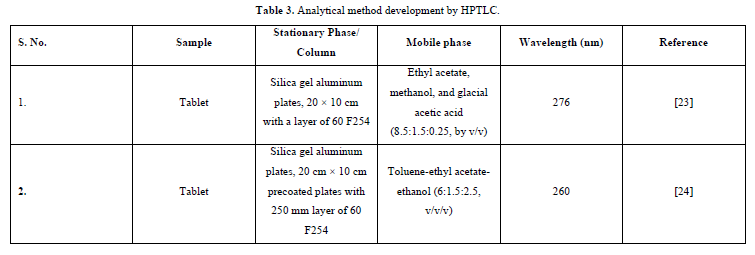
It is a powerful analytical method that is effective for both qualitative and quantitative applications. Separation may result from partitioning, adsorption, or both, depending on the variety of adsorbents used on the plates and the development solvent system. HPTLC fundamentals include a number of aspects, including principle, theory, instrumentation, implementation, optimization, validation, automation, qualitative and quantitative analysis [22] (Table 3).
BIOANALYTICAL METHOD DEVELOPMENT
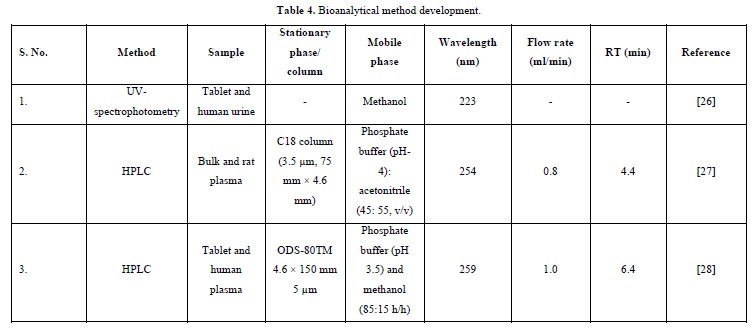
Evaluation and interpretation of bioequivalence, PK, and toxicokinetic studies are greatly aided by bioanalysis, which is used to quantify medicines and their metabolites in biological fluids. For pre-clinical and/or biopharmaceutics and clinical pharmacology studies to be effective, sensitive and selective analytical techniques for the quantitative assessment of medications and their metabolites are essential [25] (Table 4).
CONCLUSION
The primary focus of this study has been on the numerous analytical and bioanalytical techniques used to estimate the amount of etodolac in different medications and in the pharmaceuticals' bulk form. There are several dosage formulations that contain etodolac in combination. UV spectrophotometry, LC, HPLC, HPTLC, RP-HPLC, TLC, and other hyphenated procedures are among the analytical and bioanalytical techniques that the researchers have worked to create. Among the hyphenated techniques are LC-APCI/MS/MS, HPLC-MS/MS, and LC-MS/MS. Every analytical technique created is very accurate, reproducible, sensitive, automated, and has a better sample throughput. The purpose of the literature review is to gather data on various analytical instrumental techniques. Such information would be useful in creating a new analytical technique.
COMPETING INTEREST
The author reported no conflict of interest in this article.
No Files Found
Share Your Publication :