-
Publish Your Research/Review Articles in our High Quality Journal for just USD $99*+Taxes( *T&C Apply)
Offer Ends On
Khadija Rouaz-El Hajoui*, Blanca Chiclana-Rodríguez, Anna Nardi-Ricart, Marc Suñé-Pou, Débora Mercadé-Frutos, Josep María Suñé-Negre, Pilar Pérez-Lozano and Encarna García-Montoya
Corresponding Author: Khadija Rouaz-El Hajoui, Department of Pharmacy and Pharmaceutical Technology and Physical Chemistry, Faculty of Pharmacy and Food Sciences, University of Barcelona, Av. Joan XXIII, 27- 31, 08028 Barcelona, Spain.
Received: July 14, 2023 ; Revised: July 23, 2023 ; Accepted: July 26, 2023 ; Available Online: August 10, 2023
Citation: Rouaz-El-Hajoui K, Chiclana-Rodríguez B, Nardi-Ricart A, Suñé-Pou M, Mercadé-Frutos D, et al. (2023) Formulation of Omeprazole in the Pediatric Population: A Review. J Pharm Sci Drug Discov, 2(1): 1-14.
Copyrights: ©2023 Rouaz-El-Hajoui K, Chiclana-Rodríguez B, Nardi-Ricart A, Suñé-Pou M, Mercadé-Frutos D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Views & Citations
Likes & Shares
This systematic study aimed to critically review the use of omeprazole in the Pediatric population. This drug is well known and widely used but remains difficult to use in this population due to formulation and administration issues. On the one hand, this study aimed to provide an overview of the physicochemical, pharmacokinetic and pharmacological aspects of omeprazole and the issues related to extemporaneously prepared formulations, especially instability issues. On the other hand, a review of published Pediatric formulations containing this active pharmaceutical ingredient (API) was also carried out to explore how researchers have tried to solve these problems and whether they have considered stability issues. In conclusion, it seemed clear from our investigation that the necessary gastro-resistance is not always accounted for in formulations and that stability is highly dependent on specific factors, such as pH and humidity.
Keywords: Omeprazole, Pediatrics, Pharmacokinetics, Pharmacology and Stability
INTRODUCTION
Currently, children are still considered to be therapeutic orphans due to the lack of pharmaceutical options that are adapted to the needs of pediatric patients. These patients constitute a heteromorphic population that is characterized by constant changes throughout the maturation process. Therefore, a large number of pediatric pathologies are treated with drugs that have not been studied for their potential therapeutic use in the pediatric population. However, in recent decades, there has been a great amount of interest in the development of oral medicines for pediatric use, both for rare and common diseases [1,2]. According to the European Medicines Agency (EMA) [3,4], pediatric investigation plans should include measures to adapt the formulation of the medicinal product to be age-appropriate for the different subsets of the pediatric population. There are several aspects to consider when deciding whether the pharmaceutical design of a pediatric medicinal product is appropriate, including the following:
In addition, all studies on pediatric medicinal products must be in line with the WHO criteria, as well as the WHO’s “Make medicines child-sized” campaign and 2009 “Better Medicines for Children” initiative, to ensure that medicines for children are appropriate, safe and effective [5,6]. At the same time, the EMA also emphasizes the development of pediatric medicines that meet the abovementioned requirements, which can be found in the pediatric regulation section of the EMA website [7] and pediatric investigation plans [8].
Typically, the lack of marketed pediatric medicines affects hospital pharmacy services, where magistral formulations are used to meet the needs of special patients (i.e., pediatric, geriatric or swallowing-impaired patients). Regarding pediatric patients, oral liquid formulations are often the most suitable preparations because they allow for safe and easy dosage adjustment (according to body weight or body surface area, etc.) and avoid the need to swallow tablets or capsules. Obviously, liquid preparations that are formulated in hospital pharmacies must also be tested for quality and stability as medicinal and commercially available products. However, in practice, reliance is placed on official published information (i.e., information from the National Formulary, drug regulatory agencies, web-based bibliographies, etc.) because hospital centers do not have the capacity or resources to carry out stability or exhaustive quality controls as in the pharmaceutical industry. Thus, this lack of stability and quality studies limits the use of many medicines in special patients [2,9].
According to data provided by the Hospital Materno Infantil del Vall de Hebrón (Barcelona, Spain), omeprazole is one of the most widely used active pharmaceutical ingredients in magistral formulations for the treatment of gastrointestinal diseases in the pediatric population, a fact that has been corroborated by other international hospitals, including hospitals in Thailand [10], Morocco [11] and France [12]. In addition, omeprazole formulations that are used for pediatric patients must meet the quality and safety requirements of the EMA, FDA and WHO [2,9] which is very difficult due to the chemical instability problems that are associated with omeprazole. For this reason, it is important to develop pediatric formulations of omeprazole that address these stability issues.
This study explored the characteristics of omeprazole that limit the development of adequate pediatric formulations. Additionally, a review of published pediatric formulations containing this API (from PubMed, SciFindern, Scopus and Web of Science) was conducted to see how researchers have tried to overcome the issues with omeprazole and how they have demonstrated its stability [5-8]. Finally, it was clear from the review of published studies that the administration of omeprazole in children is an important issue; although there have been studies on administration in pediatric patients, the appropriate doses are not well established and the drug information does not provide recommendations for use in the pediatric population [9].
PHARMACOLOGICAL CHARACTERISTICS AND MECHANISM OF ACTION OF OMEPRAZOLE
Omeprazole is a proton pump inhibitor (PPI) and is one of the most widely used antisecretory drugs due to its good efficacy and the lack of significant adverse effects [13,14]. It selectively and irreversibly inhibits the H+/K+-ATPase or proton pump, which is the final step in the acid secretory pathway. Its inhibitory capacity is independent from the stimulus that triggers acid production, i.e., it reduces both basal and stimulated gastric secretion [15-17].
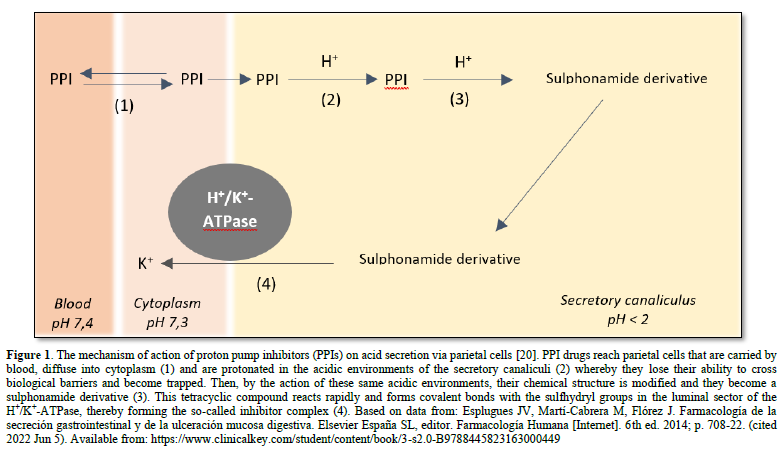
Omeprazole is a substituted benzyl imidazole derivative and a racemic mixture of two enantiomers. It should be noted that omeprazole is a prodrug that is activated in acidic media. As a lipophilic weak base (pKa of 4,0), it is electrically uncharged and highly lipid soluble at approximately pH 7, which is why it can easily cross cell membranes [15,18]. It accumulates selectively in the acidic environments of the canaliculi of stimulated parietal cells. Once it reaches parietal cells, it crosses the cell membranes via passive diffusion. In the secretory canaliculi of these cells, omeprazole is in its active achiral form and is exposed to a pH of less than 2,0 (about 1). It is ionized through a protonation process, which transforms it into a sulphonamide (i.e., a stable molecule at acidic pH levels and not lipophilic). Its positive charge prevents it from crossing parietal cell membranes, leading to the accumulation and concentration of the drug in the canaliculi [18]. This accumulation is essential as it allows for a prolonged therapeutic effect despite the drug having a short plasma half-life (Figure 1) [15]. This sulphonamide reacts by forming covalent bonds with the sulfhydryl (thiol) groups of the cysteine radicals on the extracellular surfaces of its alpha-subunits and the H+/K+-ATPase, thereby irreversibly inhibiting the activity of this enzyme. Therefore, the reactivation of the secretory activity is only possible after the resynthesize of the inhibited enzyme, which has a half-life of about 18 h [18]. This need for de novo enzyme genesis enables a prolonged inhibitory effect on acid secretion [15,16,19].
Omeprazole was originally approved by the FDA in 1989 for the treatment of gastric acid-related disorders, such as gastro-esophageal reflux, peptic ulcer disease and other conditions that are characterized by excessive gastric acid secretion. Omeprazole is generally effective and well tolerated, which has promoted its common use in children and adults. It was the first clinically useful drug of its kind and its formulation was followed by the formulation of many other proton pump inhibitor drugs [13,19,21]. The most commonly used PPI drugs are omeprazole, lansoprazole, pantoprazole (in sodium salt form), rabeprazole and esomeprazole (which is an optical isomer of omeprazole) [13,17]. Omeprazole is a white or off-white crystalline powder, which melts at 155 ºC with decomposition, has a weak basic character and is freely soluble in lipids, ethanol and methanol, slightly soluble in acetone and isopropanol and very slightly soluble in water. Its stability is pH-dependent as it degrades rapidly in acidic media but remains practically stable in alkaline conditions [19,22-24].
PHARMACOKINETIC CHARACTERISTICS OF OMEPRAZOLE
In adults, omeprazole is administered orally in the form of capsules that contain enteric-coated (pH-sensitive) mini-granules or pellets in order to prevent ionization by acidic gastric environments and promote absorption in the duodenum (which increases its bioavailability by up to 50%). There are also buffered release forms of omeprazole and preparations for intravenous administration [13,15].
The absorption of omeprazole depends on the different formulations and therefore, its oral bioavailability is also varied. It should be noted that its bioavailability is the same whether administered orally or via a nasogastric tube [18,25].
After absorption in the small intestine, omeprazole passes from the blood into the parietal cells of the stomach and then into the canaliculi, where it exerts its therapeutic action [15]. It is metabolized in the liver by the cytochrome P450 enzyme complex, which is an enzyme that is absent in approximately 3% of white people and 20% of Asian people, hence why there may be more cases of intoxication when using the drug in the usual doses [15,26]. The CYP2C19 and CYP3A4 isoenzymes are responsible for most of its metabolism; therefore, changes in the maturation of this enzyme complex may affect the pharmacokinetic parameters of omeprazole. Cytochrome P450 activity is low at birth, reaches adult levels in early life and then increases and surpasses adult levels during childhood before recovering to adult levels after puberty [16]. Parietal cell immaturity and achlorhydria during the first 20-30 months of life are also maturation-dependent factors for the two isoenzymes that may prevent the transformation of omeprazole into its active form and, consequently, its accumulation in the intracellular canaliculi of parietal cells. Gastric emptying and intestinal transit are other age-varying factors that may affect the bioavailability of omeprazole in the pediatric population [16,27,28]. Thus, in order to determine the most appropriate dose for different age groups, safety and efficacy studies must be conducted.
As a consequence of the particular mechanism of action of omeprazole, the level of acid inhibition that is produced does not correlate with the plasma concentration but instead with the area under the plasma concentration versus time curve. This is because omeprazole forms covalent bonds with the proton pump stimulating enzyme, which means that the therapeutic effect of a single dose of the drug is maintained for more than 24 h even though its plasma half-life is 60 min [24]. The administration of food delays absorption and decreases the area under the plasma concentration versus time curve. Therefore, omeprazole should be administered on an empty stomach, preferably first thing in the morning, regardless of the time elapsed between the administration of the drug and the subsequent ingestion of food [13,18].
The initial bioavailability of omeprazole is low, so its maximum effect is not reached with the first dose but instead after 5-7 days of repeated administration. This gradual build-up could be due to the fact that gastric secretion is inhibited after the first few doses, leading to a decrease in the gastric degradation of successive doses. Alternatively, it may be due to reduced first-pass metabolism. Omeprazole is more than 90% bound to plasma proteins (mainly albumin and α1-acid glycoprotein), so its volume of distribution is low (0,3-0,4 L/kg). It can cross the blood–brain and placental barriers [13,15].
CLINICAL TRIALS ON THE PHARMACOKINETICS OF OMEPRAZOLE IN PEDIATRICS
The pharmacokinetics of omeprazole has been studied in children but mainly in those older than 2 years, so studies on younger children are still needed [29]. In a multicenter study by Andersson [30], the pharmacokinetics of orally administered omeprazole was evaluated in children from different age groups (1-16 years). The authors concluded that the pharmacokinetic parameters were mainly in the same range as those for adults, with the exception of the 1-6-year age group in which increased metabolic activity was observed. These results suggested an increase in metabolic activity as age decreased until the second year of life. This increased metabolic capacity in children aged 1-6 years is probably the main factor that explains the higher omeprazole dose requirements for young children compared to older children and adults.
In a subsequent study, also by Andersson et al., the pharmacokinetics of intravenously administered omeprazole was evaluated in new-born and infants requiring acid suppression [31]. The half-life and clearance of omeprazole in neonates (≤ 10 days) was found to be longer and lower than those in children aged 4,5-17 months. These values for the half-life and plasma clearance (expressed per body weight) could be explained by the low CYP2C19 and CYP3A4 enzyme activity that is present at birth. It is worth mentioning that these values were also close to the values of slow metabolizers with respect to the CYP2C19 enzyme. It was concluded that the new-born had a lower omeprazole metabolization rate than the other infants in the study, suggesting that their CYP2C19 and CYP3A4 enzymes were not yet fully mature.
It is worth highlighting that omeprazole is widely used for the treatment of gastric acid-mediated disorders. However, its pharmacokinetic and chemical instability does not allow for the synthesis of simple aqueous dosage formulations for the treatment of special patients (i.e., pediatric, geriatric or swallowing-impaired patients). Therefore, Karami [32] conducted a randomized parallel pilot study involving 34 pediatric patients with acid peptic disorder, who were treated with omeprazole. An omeprazole suspension was prepared by adding omeprazole powder to 8,4% sodium bicarbonate to obtain a final concentration of 2 mg/ml of omeprazole. Patients received either the suspension or granules. After oral administration, blood samples were collected and analyzed for omeprazole levels using a validated HPLC method. No significant differences were observed between the two dosage forms, both 2 h before and after the last dose. These results demonstrated that omeprazole suspensions are a suitable substitute for granules in the pediatric population [32].
In another prospective randomized clinical trial involving critically ill children who were at risk of gastrointestinal bleeding, Solana et al. studied the effects of two doses of intravenously administered omeprazole on gastric pH and the incidence of gastrointestinal bleeding. It was established that therapeutic efficacy was achieved when the gastric pH was above 4 and there was an absence of clinically significant gastrointestinal bleeding [33]. Between 24 and 48 h, a 1 mg/kg dose maintained gastric pH above 4 for a greater amount of time. The plasma levels of omeprazole were found to be higher after the 1 mg/kg dose. However, no correlations were found between omeprazole plasma levels and gastric pH levels. No toxic adverse effects were detected and there was no clinically significant bleeding [33].
Hassall [34] conducted an open multicenter study involving 57 children aged from 1 to 16 years old to determine the efficacy, safety and tolerability of omeprazole as a treatment for erosive esophagitis among this group of patients. For the curative dose of omeprazole, the investigators used a dose that corresponded to the treatment of acid reflux of less than 6% in a 24-hour intra-esophageal pH study. The dose correlated with the degree of esophagitis but not with age or underlying diseases. Of the 57 patients who completed the study, two thirds had grade 3 or 4 (0-4 scale) chronic esophagitis and about half had a neurological impairment or repaired esophageal atresia. It was concluded that omeprazole was well tolerated, effective and safe when used as a treatment for erosive esophagitis and gastro-esophageal reflux symptoms in children, including those for whom anti-reflux surgery or other treatment had failed. Reflux symptoms improved dramatically in almost all patients, including uncured patients. Additionally, the doses per Kg of omeprazole that were needed to cure erosive esophagitis in children were found to be higher than those needed in adults: 0,7-3,5 mg/kg/day in 44% of patients and 1,4 mg/kg/day in another 28% of patients.
A study by Strauss [35] investigated the effect of omeprazole on refractory histological esophagitis in pediatric patients. In total, 18 patients with histological esophagitis and recurrent symptoms who had been treated with H2-receptor antagonists and prokinetic agents were prospectively treated with omeprazole. It was well tolerated in most patients and no short-term adverse reactions were observed. In patients with only histological evidence of esophagitis, omeprazole doses of approximately 0,5 ± 1,0 mg/kg/day were useful in controlling symptoms and improving esophageal histology. Those doses were similar to those used in adults with erosive esophagitis. Omeprazole did not produce prolonged symptomatic remission in children with recurrent esophagitis, even after the documented healing of esophagitis. No advantages were found for using omeprazole in patients whose symptoms were previously controlled by H2-receptor antagonists. It was concluded that the treatment of symptoms with omeprazole could be advisable for patients without erosive esophagitis. However, the long-term progression of histological esophagitis could not be determined.
ADVERSE EFFECTS OF OMEPRAZOLE
The introduction PPIs in 1989 marked a turning point in the treatment of heartburn-related disorders. Due to their novel and effective mechanism of action and low side effects, these drugs rapidly displaced others (e.g., H2 antagonists), leading to an exponential increase in their prescription. However, this widespread use of PPIs has led to evidence of some previously undescribed adverse effects, particularly in the long term. Below is a summary table (Table 1) describing the adverse effects associated with the use of PPIs and investigated in recent decades.
Table 1. Adverse effects of omeprazole.
|
Adverse effect |
Study |
Conclusion |
Reference |
Year |
|
Association between PPI use and risk of pneumonia in children |
Self-controlled case series study |
An increased risk of pneumonia was observed both immediately before and immediately after starting PPI treatment. This pattern of association could probably be explained by the underlying risk of pneumonia due to factors that were transiently present around the time PPI treatment initiation. In this case, it was concluded that the obtained results did not support a causal relationship between PPI use and pneumonia risk |
[36] |
2022 |
|
Association between PPI use and the risk of depression and anxiety |
Cohort study |
PPI use was associated with an increased risk of depression and anxiety in children |
[37] |
2022 |
|
Increased risk of renal, liver and cardiovascular disease, dementia, enteroendocrine tumors in the gastrointestinal tract, susceptibility to respiratory and gastrointestinal infections, and impaired nutrient absorption |
Review |
The risks and benefits of long-term PPI use should be carefully considered, especially in young patients whose treatment with these drugs could last for many years |
[38] |
2021 |
|
Association between PPI use and the risk of asthma in children |
Cohort study |
PPI use was associated with an increased risk of asthma in children compared to non-use |
[39] |
2021 |
|
Myocardial infarction, stroke, miscarriage, spontaneous abortion, proliferative changes, chills, heart failure, thrombosis and dementia |
Review (72 articles) |
The use of omeprazole should be monitored in patients with cardiac disorders using concomitant antiplatelet agents and patients with new transplants using mycophenolic acid to avoid serious adverse reactions. |
[40] |
2018 |
|
Effect of long-term omeprazole therapy on the numbers of antral G and D cells in children |
Review |
Omeprazole therapy was associated with a significant increase in the number of G cells and the ratio of G to D cells in children |
[41] |
2001 |
|
Increased risk of acute gastroenteritis and pneumonia in children treated with gastric acidity inhibitors |
Multicenter prospective study: 186 subjects |
The number of subjects presenting with acute gastroenteritis and community-acquired pneumonia was significantly higher in patients who were treated with GA inhibitors compared to the healthy controls during the 4-month follow-up period |
[42] |
2006 |
|
Effects of PPI use on duodenal bacteriology, carbohydrate absorption and bowel habits |
Review |
Conventional treatment for duodenal ulcers with a PPI significantly increased the bacterial colonization of the duodenum and intestinal transit speed |
[43] |
1996 |
Although most researchers claim that more studies are needed, it seems clear that the most common side effects of omeprazole therapy are asthma [39], pneumonia [36,42] and acute gastroenteritis [42].
PEDIATRIC OMEPRAZOLE FORMULATIONS
It is important to note that there have not been many published studies concerning pediatric formulations of omeprazole, even though it is one of the most commonly prescribed APIs in the pediatric population.
With respect to the stability of omeprazole as a raw material, it is a substance that can be degraded by several factors, which have to be considered when developing formulations. Some of these factors are discussed in Table 2 [44-47]. It is clear that the formulation of this drug is not straightforward.
In order to facilitate our understanding of the current state of the subject, two summary tables (Tables 3 & 4) were drawn up following the literature search. Table 3 shows a list of published omeprazole formulations that are suitable for the pediatric population, although they are in the early stages of development. Most have undergone tentative quality controls to demonstrate the chemical validity of the product; however, most of the stability studies that have been carried out to demonstrate how long the proposed formulation is stable have not considered the gastro-resistance test. As mentioned previously, omeprazole is sensitive to acidic environments and needs to be protected from these environments so that it can pass through the cells of the intestine into the blood.
Table 2. External factors that affect omeprazole stability.
|
Factor |
Effect of factor on the stability of omeprazole |
Reference |
|
pH |
Depends on the pH of the solution. At 20ºC, the half-life of the product is 15 minutes at pH 4 and only 1.8 minutes at pH 2. At pH 7, the half-life is about 30 hours while at pH 9, it is more than one week. At 37°C, the half-life is about 10 hours at pH 7, while at alkaline pH levels in a 0.1 N sodium hydroxide solution, it is about one year. |
[44-47] |
|
Temperature |
Omeprazole is virtually stable at elevated temperatures (37ºC to 50ºC). A slight discoloration has been observed in samples stored at elevated temperatures. |
|
|
Ultraviolet Light |
After exposure to artificial light (xenon lamp: 280-830 nm; approximately 830 W/m2; 150 000 lux) for 48 hours, omeprazole was degraded by 20% in one study. The main degradation product was 5-methoxy-2-mercaptobenzimidazole, which is one of the starting products in the synthesis of omeprazole. When samples were protected by amber-colored glass, the stability of omeprazole increased considerably. |
|
|
Humidity |
It is recommended to store omeprazole in airtight packaging to avoid the action of external factors (humidity, oxygen, etc.). If omeprazole is not protected from humidity, it changes from its initial white color to a brownish color and may even turn black in the case of exposure to extreme humidity. Omeprazole specialties that are repackaged using heat-sealing systems that do not adequately protect against moisture have a stability time of 7 days when stored under ambient conditions. |
This is a key point in the development of omeprazole formulations. In Table 3, the formulations are grouped according to their pharmaceutical form: suspensions, syrups, mucoadhesive tablets, mucoadhesive films and suppositories. All of the formulations were considered to be good alternatives to the extemporaneous oral omeprazole preparations that are produced as officinal or compounding formulas in hospitals, most of which are made by manipulating commercial omeprazole drugs (capsules with pellets or mini-granules) or using omeprazole powder in sodium bicarbonate solutions. If these proposed alternatives are confirmed, they could improve the therapeutic efficacy and facilitate the administration of this active pharmaceutical ingredient in the pediatric population.
Further examples of stability studies on the liquid compounding formulas and extemporaneous preparations of omeprazole that are used in the pediatric population are presented in Table 3. Among the presented examples, the following parameters were generally studied: the shelf life of the preparations and the optimal storage temperature. It was observed that most preparations are best stored refrigerated rather than at ambient temperatures. It is noteworthy that only three of the studies (examples 3, 5 and 6 in Table 4) found that the solution turned yellow after 7 days, which is quite common when working with omeprazole [48-50]. More specifically, it is interesting to note that the recapitulated examples studied the stability of omeprazole suspensions at different concentrations [48,51]. In example 3, it was concluded that suspensions comprising sodium bicarbonate and 0,6-4 mg/mL of omeprazole could be stored at 4ºC in the dark for up to 28 days [48]. Other parameters, such as viscosity (example 3) and pH (examples 5 and 6), were also studied [48-50]. None of the examples studied the gastro-resistance required for omeprazole in depth or how the preparation of an alkaline liquid affects the protection of omeprazole once it reaches the stomach. For example, if the patient is able to drink, a common practice is to administer pellets with an acidic drink (e.g., fruit juice) to avoid the action of gastric juice with a pH of less than 5.3 [18]. This practice is also not discussed in the various studies collected.
Table 3. Examples of pediatric omeprazole formulations.
|
Pharmaceutical Form |
Excipients |
Controls |
Stability |
Reference |
Year |
||
|
Liquid Preparations |
Suspensions |
|
Sesame oil (37.5%; carrier) Compritol® (1.54%; viscous agent) |
Very good palatability (beige color, pleasant smell with vanilla and caramel aromas, sweet taste and creamy texture) Good content uniformity (by shaking before use) |
|
|
|
|
|
Soy lecithin (0.1%; emulsifying and stabilizing agent) |
Good release profile |
Store in a refrigerated and amber-colored container |
|
|
||
|
Example 1: 2% omeprazole oily suspension |
Calcium carbonate (14.5%; protective antacid) |
|
|
|
2012 |
||
|
|
|
|
|
[52,53] |
-2015 |
||
|
|
|
|
Aspartame (0.11%; sweetener) |
|
|
|
|
|
Labrafac (42%; omeprazole suspension vehicle) Vanilla and caramel |
|||||||
|
flavorings (0.05%) |
|||||||
|
Liquid Preparations |
Suspensions |
|
Eudragit® RS 100 (extended- release polymer with a nanoparticle matrix) Eudragit® L 100-55 (pH- sensitive gastro-resistant polymer) |
|
|
|
|
|
|
Acetone (solvent) Peanut oil (to promote the formation and loading of the |
|
|
|
|
||
|
|
API into nanoparticles) Polysorbate 80 (surfactant) Sodium bicarbonate (pH regulator) |
|
|
|
|
||
|
|
NaOH solution 0.03 M (alkaline excipient) |
|
|
|
|
||
|
Example 2: Enteric-coated omeprazole nanoparticle suspension (0.5 mg/mL) |
|
|
|
|
|
||
|
|
|
The release of enteric omeprazole nanoparticles is pH-dependent |
Storage and stability conditions are not specified |
|
|
||
|
|
|
|
|
[54] |
2020 |
||
|
Liquid Preparations |
|
|
Excipient Coating (by fluid bed) |
|
|
|
|
|
|
|
Microcrystalline cellulose pellets (diameter of 200–300 µm) |
|
|
|
|
|
|
|
|
Eudragit® L100-55 (enterosoluble polymer) Eudragit® E100 (gastrosoluble polymer) Povidone (binder) |
|
|
|
|
|
|
|
|
Talc (10 µm of micronized talc; binder) |
|
|
|
|
|
|
|
|
Ascorbic acid palmitate (antioxidant) |
|
|
|
|
|
|
|
|
Hydrogen phosphate (antioxidant) |
|
|
|
|
|
|
|
|
Disodium hydrogen phosphate dihydrate (pH regulator) Silicon (silicon emulsion) Silicon (30% simethicone emulsion; anti-foaming agent) Titanium dioxide (opacifier) Tryethyl-2-acetylcitrate (plasticizer) |
|
|
|
|
|
|
|
|
-Glyceryl monostearate (hydrophobic excipient to increase the strength of the polymer against water) Aluminum oxide (excipient with anti-electrostatic properties) |
|
|
|
|
|
|
|
|
Excipient Syrup Sorbitol (syrup vehicle; sweetener and stabilizer) |
|
|
|
|
|
|
|
|
Microcrystalline cellulose and sodium carboxymethylcellulose (viscous agents) Polyvinylpyrrolidone (syrup vehicle; suspending agent; sweetener and stabilizer) Sodium carbonate anhydrous and disodium hydrogen |
|
|
|
|
|
|
|
|
phosphate dihydrate (pH neutralizers) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Example 3: Delayed- release liquid in oral dosage form based on omeprazole (2 mg/mL) |
|
|
|
|
|
|
|
|
|
|
Delayed-release properties |
Stable after 10 days at room temperature |
|
|
|
|
Syrups |
|
|
-Multi-layered diameter of less than 500 µm (to avoid swallowing problems) |
|
[55] |
2019 |
|
|
|
|
Example 4: Adhesive buccal |
|
Physicochemical properties, including bio- adhesive strength |
Storage and stability conditions are |
|
|
|
Solid Preparations |
Bucoadhesive Tablets |
Sodium alginate (bio-adhesive polymer) |
not specified |
[56] |
2000 |
||
|
|
|
omeprazole tablets (20 mg) |
Hydroxypropyl methyl cellulose (bio-adhesive polymer) |
23% of the administered omeprazole dose is absorbed into the oral cavity within 15 minutes |
Stable in human saliva for 4 hours |
|
|
|
Carbopol and polycarbophil (cross-linking agents; anionic and water swellable polymers) Monosodium potassium phosphate, monobasic sodium phosphate, dibasic sodium phosphate and magnesium |
|||||||
|
oxide (alkaline excipients) |
|||||||
|
Solid Preparations |
|
|
Carrageenan (gel base and stabilizing agent) |
|
|
|
|
|
|
Example 5: Pediatric omeprazole: L- arginine films (ratio 1:2) |
Sodium alginate (bio-adhesive polymer) |
|
|
|
|
|
|
|
|
Metolose® (viscous agents) Polyethylene glycol 400 (plasticizer) |
Good molecularly dispersion within the Metolose® film matrix |
Storage and stability conditions are not specified |
|
|
|
|
Oral Films |
|
L-arginine (pH stabilizer) Ethanol and water (solvents) |
|
|
[57] |
2014 |
|
|
Solid Preparations |
|
|
Metolose® (mucoadhesive polymer) |
|
|
|
|
|
|
|
Polyethylene glycol 400 (plasticizer) |
|
Stable at room temperature for 28 days |
|
|
|
|
|
|
L-arginine (stabilizer) Gelatine (gelling and film- forming agent) |
|
More stable at room temperature than at 40°C |
|
|
|
|
|
|
Beta- and gamma- cyclodextrin (stabilizers and release modulators) |
|
Short and long- term stability improves with increased pH levels |
|
|
|
|
|
Example 6: Paediatric omeprazole oral films |
Ethanol (solvent) Potassium dihydrogen phosphate and sodium |
|
|
|
|
|
|
Oral Films |
|
hydroxide (pH regulators) |
No controls specified |
|
[58] |
2018 |
|
|
Solid Preparations |
|
|
Tetraethyl orthosilicate (type SBA-15; mesoporous silicate) Sodium alginate (matrix- forming agent for gel microspheres) |
Uniform size distribution and drug content |
|
|
|
|
|
Example 7: Alginate microspheres as vehicles for omeprazole/SB A-15 |
Pluronic® 12 3 (surfactant) Granular anhydrous calcium chloride (aqueous solution; |
Drug dosage ranges from 1% to nearly 7% w/w Homogeneous and reproducible release kinetics |
|
|
|
|
|
|
|
cross-linking agent) Deionized water (solvent) |
All formulations demonstrate enteric properties, except one |
Storage and stability conditions are not specified |
|
|
|
|
Alginate Microspheres |
|
|
|
|
|
|
|
|
|
|
|
|
|
[59] |
2015 |
|
|
Solid Preparations |
|
|
|
|
Omeprazole content is stable at 90-110% for |
|
|
|
|
|
|
|
1 year when stored in the dark at room temperature A long-term stability study |
|
|
|
|
|
|
|
|
showed no signs of discoloration |
|
|
|
|
|
Example 8: Omeprazole suppositories for children |
L-arginine base (stabilizer) Witepsol® H15 (melting wax; base of suppository) |
|
|
|
|
|
|
Suppositories |
|
|
No controls specified |
|
[60] |
2020 |
|
Table 4. Examples of stability studies on the liquid compounding formulas and extemporaneous preparations of omeprazole that are used in pediatrics
|
Formula |
Composition |
Study and Storage Conditions Results: Stability |
Reference |
Year |
|
||
|
Liquid Preparations |
Example 1: Oral liquid omeprazole suspension for pediatric patients (2 mg/mL) |
Formulation A: Oral liquid omeprazole suspension (2 mg/mL) using crushed omeprazole granules Formulation B: Oral liquid omeprazole suspension (2 mg/mL) using an omeprazole base with a complete vehicle, including wetting agents, suspending agents, sweeteners, antioxidants and flavorings |
Formulations A and B can be stored for at least 150 and 90 days, respectively, under refrigerated conditions (4 ºC) Formulation A can be stored at room temperature (25 ºC) for 14 days, while Formulation B is not recommended to be stored at room temperature for more than 1 day |
[61] |
2020 |
|
|
|
|
Example 2: Omeprazole suspension from the contents of 20 mg omeprazole capsules (2 mg/mL) |
Formulation: A 2 mg/mL omeprazole suspension was prepared by emptying 10 omeprazole capsules (20 mg) and mixing with 1 g of methylcellulose. Then, a sodium bicarbonate solution |
Self life is 32 days at room temperature and 54 days when refrigerated (2-8ºC) |
[62] |
2018 |
||
|
(8.4%) was added and homogenized to 100 mL with the same solution |
|||||||
|
|
|||||||
|
Liquid Preparations |
Example 3: Omeprazole and sodium bicarbonate suspension (2 mg/mL) |
Formulation A: A commercial preparation of 20 mg of omeprazole and sodium bicarbonate suspended at initial omeprazole concentrations of 0,6 and 2 mg/mL |
|
[48] |
2006 |
||
|
Formulation B: A commercial preparation of 40 mg of omeprazole and sodium bicarbonate suspended at initial omeprazole concentrations of 1,2, 2, 3 |
Suspensions were stored at 4ºC in the dark (refrigerated) or at 22-25ºC (room temperature) in the light for one week (samples were also stored refrigerated for 1 month) |
||||||
|
and 4 mg/mL |
Suspensions of 0,6-4 mg of omeprazole per mL can be stored at 4 °C in the dark for up to 28 days |
||||||
|
Stability of omeprazole was quantified using HPLC |
No viscosity variations over 7 days Samples contained 90% of their initial omeprazole content after 7 days despite turning yellow |
||||||
|
Viscosities of refrigerated suspensions were measured after 0, 1 and 7 days |
|
||||||
|
|
|
||||||
|
Liquid Preparations |
Example 4: Commercial omeprazole and sodium bicarbonate powder suspension |
Formulation: An omeprazole/sodium bicarbonate suspension (2 mg/mL) Samples were stored refrigerated and analyzed by HPLC immediately after preparation and after 7, 15, 30 and 45 days |
|
|
|
||
|
The stability of a 1 mg/kg dose was determined with an estimated volume of simulated gastric fluid for a hypothetical 12.7 kg pediatric patient in |
|
||||||
|
triplicate over 2 hours at 37 ºC |
The suspension was stable for at least 45 days when stored at 3-5 ºC |
||||||
|
|
A partial dose of 12.7 mg was stable following exposure to simulated gastric fluid for 2 hours at 37 ºC |
||||||
|
|
|
||||||
|
Liquid Preparations |
Example 5: Omeprazole suspension from commercial 20 mg capsules (2 mg/mL) |
|
Samples were stored in 100 mL amber glass bottles under refrigeration (2–8 ºC) or at room temperature (21 ± 2 ºC) One of each sample was shaken and the other was not shaken, then the pH was measured and color changes were determined using a visual scale Omeprazole concentrations were measured using tandem mass spectrometry with liquid chromatography |
[49] |
2008 |
||
|
|
Formulation A was stable for 45 days in a refrigerator when shaken regularly (without shaking, API decreased rapidly for 7 days) |
||||||
|
|
Color changes were observed in the samples |
||||||
|
|
pH remained constant |
||||||
|
Formulation A: Oral liquid omeprazole suspension (2 mg/mL) using the contents of Probitor® (20 mg omeprazole) capsules |
|
||||||
|
Formulation B: Oral liquid omeprazole suspension (2 mg/mL) from adding omeprazole powder to 8.4% sodium bicarbonate solution |
|
||||||
|
|
|
||||||
|
|
|
||||||
|
Liquid Preparations |
Example 6: Omeprazole suspension (2 mg/mL) |
Formulation: Omeprazole base (0.2%), sodium bicarbonate (8,4%), Xanthan gum 1% aqueous solution (50 mL), vanilla essence (0.1-0.2%), saccharin sodium (0.1-0.3%) and purified water (qsp 100 mL) |
The pH ranges studies were 1.2, 2.2 and 4.5 |
[50] |
2021 |
||
|
10 mL of the suspension, equivalent to 20 mg of omeprazole, was added to each of the pH media and observed for 2 hours |
|||||||
|
10 mL of a placebo was also added to a |
|||||||
|
1.2 pH medium and observed for 2 hours |
|||||||
|
At pH 1.2, a change in color from clear to slightly yellow was observed after less than 1 minute, which intensified after 5 minutes |
|||||||
|
At pH 2.2 and 4.5, the samples were no longer completely transparent and began to show slight color changes after 5 minutes |
|||||||
|
The placebo medium did not change color |
|||||||
|
|
|||||||
|
|
|
||||||
|
|
|
|
The 2 mg/mL omeprazole suspension was instable in acidic media (the color changes indicated the degradation of |
|
|
||
|
the omeprazole) |
|||||||
|
Liquid Preparations |
Example7: Omeprazole suspension (2 mg/mL) |
Three oral suspensions of omeprazole were prepared using omeprazole powder at three concentrations (2, 5 and 10 mg/ml) and Oral Mix Dry Alka, SF (OMSF®), which contains calcium carbonate as a pH neutralizing agent, was used as the suspension vehicle |
The concentration of omeprazole was determined after 0, 7, 14, 28, 42, 56 and 70 days using HPLC with photodiode array detection (HPLC-PDA) |
[51] |
2022 |
||
|
The pH, homogeneity, color, odor and microbial levels were also determined Omeprazole was stable in the OMSF® vehicle for 70 days |
|||||||
|
Preparations were stable at 4ºC for 70 days |
|||||||
|
The pH decreased from 9.0 to 7.7 during the study, which was an acceptable change |
|||||||
|
The microbiological study was correct None of the samples showed any changes in color, odor or appearance |
|||||||
|
over 70 days |
|||||||
|
|
|||||||
|
|
|||||||
DISCUSSION
Omeprazole is widely used for the treatment of gastric disorders in the pediatric population. However, as previously mentioned in this review, the main problem with this active pharmaceutical ingredient (API) is its degradation in acidic environments [32,58]. This point is crucial because gastro-resistant excipients are not recommended for the pediatric population unless their use is completely justified [2]. Furthermore, omeprazole doses are not well established for pediatric population and the drug information does not include any recommendations for children [9]. Thus, hospitals do not have any guidelines for the administration of this medicine in pediatric patients.
Regarding the instability of omeprazole in acidic media, formulators are used to ensure the release of the API in the small intestine, thereby protecting omeprazole from gastric pH levels. One alternative that healthcare professionals apply to administer omeprazole to the pediatric population is the use of nasogastric tubes, which deliver the API directly to the small intestine [18,25]. Another common practice is the administration of omeprazole powder with sodium bicarbonate to promote alkaline pH levels [63]. However, this strategy seems to degrade the API (i.e., the solutions turn yellow) and the release of omeprazole in the small intestine has not been demonstrated. The third most widely used strategy is the preparation of liquid formulations that incorporate gastro-resistant pellets from commercialized capsules in acidic drinks (i.e., fruit juices). The acid pH levels of those drinks prevent the release of omeprazole in the liquid formulation. However, none of these preparations and strategies have shown the same quality or stability parameters as commercial products. Indeed, there is a lack of information about their stability [2,9]. There have only been a few studies describing the stability of some of these preparations. For example, there have been studies demonstrating that alkali solutions with gastro-resistant pellets are stable for 7-32 days [48,49,61,62] or 28 days [58] at room temperature.
In this study, a review of articles describing pediatric formulations of omeprazole was performed. Examples of various dosages and forms that are suitable for use in the pediatric population were collected, including suspensions, syrups, oral tablets, mucoadhesive films and suppositories. These examples could be good alternatives to the current officinal or extemporaneous preparations of omeprazole. Nevertheless, most of these works did not demonstrate the gastro-resistance of the proposed formulations (the only work that did discuss this point was the study by Del Gaudio et al. [59]). Furthermore, the stability problems were not fully resolved in these studies. Indeed, most of the proposed preparations had to be stored refrigerated [52,53,63] and were only stable for 2-4 weeks. The formulation that demonstrated the longest stability (1 year) was the suppository developed by Bestebreurtje [60]. Thus, more research is needed to solve the gastro-resistance and stability issues of omeprazole formulations.
In recent years, multiparticulate and orally disintegrating tablet (ODT) formulations have attracted interest, along with liquid formulations [64]. However, the aim is to develop final dosage forms without compromising the pharmaceutical efficacy of the drug [64]. Another interesting approach is the development of microspheres [59] or nanoparticles [54] that can encapsulate the API. However, studies on these drug delivery vehicles have not demonstrated the gastro-resistance of the developed formulations. Currently, new technologies (such as additive manufacturing (AM), commonly known as 3D printing (3DP)) are opening new frontiers in pharmaceutical applications. For example, 3DP is a tool that enables the manufacture of formulations with intricate structure designs, customized dosing and drug combinations and controlled release. Hence, 3DP allows for the customization of medicines, which could revolutionize pharmaceutical practice and provide personalized medicines for the pediatric population [65].
CONCLUSION
Omeprazole is a drug that has demonstrated its efficacy and security in adult patients over the last 30 years. However, the safe administration of omeprazole in children has not yet been fully resolved; although it has been studied at the clinical level and its therapeutic usefulness for the pediatric population has been validated. Nevertheless, the problems of its stability in acidic media and the need to adapt the dose according to the weight of the patient are barriers to its routine use. Different extemporaneous preparations are normally used but they do not solve the stability issue, as reported in this review. Clearly, much more investment and effort are required to assess all of the critical points during the development stage and more clinical trials are needed, including trials involving the pediatric population.
FUNDING
We acknowledge the financial support of the Department de Recerca i Universitats de la Generalitat de Catalunya (AGAUR 2021 SGR 01068).
García PM (2019) Pediatric pharmacology. 1.a ed. Ciudad Autónoma de Buenos Aires: Journal.
No Files Found
Share Your Publication :