-
Publish Your Research/Review Articles in our High Quality Journal for just USD $99*+Taxes( *T&C Apply)
Offer Ends On
Raghavendra Rao M V*, Srinivasa Rao D, Vijay Kumar Chennamchetty and Dilip Mathai
Corresponding Author: Raghavendra Rao M V, Scientist-Emeritus and Director Central Research laboratory, Apollo institute of Medical Sciences and Research, Hyderabad, TS, India
Received: January 24, 2021 ; Revised: February 08, 2021 ; Accepted: February 10, 2021
Citation: Raghavendra Rao MV, Srinivasa Rao D, Chennamchetty VK & Mathai D. (2021) Management of Immune Responses to Bacterial Infection.J Clin Immunol Res Ther, 1 (1): 1-8.
Copyrights: ©2021 Raghavendra Rao MV, Srinivasa Rao D, Chennamchetty VK & Mathai D. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Views & Citations
Likes & Shares
When pathogenic bacteria enter the body through wounds and cuts in the skin surface, they may be immediately ingested by phagocytes or attacked by killer cells. Many types of pathogenic bacteria are resistant to being engulfed by phagocytes because they have anti-phagocytic substances in their membranes, which prevent phagocytes from getting a grip on them.
However, once the bacterial cell is coated with antibody molecules bound to antigenic determinants on its surface, the antibodies can form a bridge between the bacteria and the phagocyte, holding the two together so that phagocytosis can take place. Thus, extracellular bacteria are eliminated by antibody-mediated defense. CMI plays an important role in extracellular bacteria. IgA interfere with the attachment molecule and prevent colonization of pathogenic bacteria. Many organisms like Diphtheria, Tetanus, and botulism produce disease through their exotoxins. Antibodies acquired by their immunization or previous infection or given passively neutralize the bacterial exotoxins.
Antibody can interfere with the normal function of bacteria. Antibody that binds to accessible antigens on the surface of bacterium together with C3b component of complement, acts as an opsonin and increases phagocytosis and clearance of the bacterium. Antitoxins are effective in so far as they block that part of toxin molecule which is responsible for its toxic properties and neutralize its effects. The protective effect of antibodies against bacterial exotoxins depends upon their being present in the body fluids in quantities sufficient to neutralize toxin elaborated by the invading microorganism faster than it is produced.
Circulating helper T cells recognize these bacterial fragments and begin to produce proteins called cytokines. Tc cells usually recognize antigens in association with class -1 MHC products, whereas helper T-cells (Th cells) recognize antigen in association with MHC class 11 products.
Keywords: Cytokines, The T-Cell response, CD4+Helper T Lymphocytes, CD8+ Cytotoxic T-Lymphocytes, immunological response, Neutralizing antibodies, B-cell memory responses, (IFN)-? -secreting T helper type 1 (Th1) cells, HLA system
INTRODUCTION
S. aureus has virulence factors that modulate components of the human innate and adaptive immune system [1].
Adhikari [2] measured serum antibodies to an array of staphylococcal exotoxins and observed that low antibody titers correlated with a higher risk for development of sepsis. It is well known that T cell responses are essential for induction of high-affinity Abs, as well as for the longevity of the Ab-producing B cells, by providing survival and differentiation signals [3] and by inducing and maintaining memory B cells [4,5].
Cytokines secreted from T cells, such as IFN-? or IL-4, are responsible for guiding the Ig classes and subclasses [6].
Th17 cells have been shown to protect against bacterial infections, such as Streptococcus pneumonia [7,8].
Assessment of the levels of antibodies against diphtheria toxin at the onset of the disease is recommended as a complementary diagnostic criterion [9].
When neutrophil elastase cleaves the flagellar hook protein, Flg E; in its absence, the anti-sigma factor Flg M accumulates inside the bacterial cell and inhibits transcription of the gene encoding flagellin, FliC [10].
Yersinia pestis, bacilli appeared to reside within CD11b- expressing macrophages for the first several days of infection [11].
In Vibrio cholerae, the B-cell memory responses also develop for the T-cell independent antigen LPS, suggesting that memory B-cell responses to some V. cholerae antigens may be T-cell dependent [12].
TLRs are the cell membrane bound PRRs which recognize the PAMPs during Campylobacter infection [13].
An earlier study by Khair et al. found an increase in the expression of ICAM-1 and cytokines tumor necrosis factor alpha (TNF-a), interleukin-6 (IL-6), and IL-8 in human bronchial epithelial cells in response to LOS stimulation [14]. It has also been found that LOS of Histophilus somni is able to engage the NF-kB transcription factor, a potent activator of the innate immune response [15].
Infection of cell lines Caco-2 and HT29 with Brucella strains does not induce the secretion of pro-inflammatory cytokines as TNFa, IL-1ß, MCP-1, IL-10, or TGF-ß; however, they produce IL-8 and chemokines CCL-20 [16].
Studies on cellular immunity to B. pertussis have shown that recovery from whooping cough in children is associated with the induction of interferon (IFN)-y secreting T helper type 1 (Th1) cells [17].
Complementary studies in mice have shown that IFN-y is required for the control of B. pertussis infection in the respiratory tract; mice lacking this cytokine or its receptor develop disseminating and lethal infection [18].
In Neisseria meningitides Antigen-specific T-cell precursors are at lower levels in neonates than in adults [19].
Less mitogen-induced interleukin-2 (IL-2), interferon-? (IFN-?), IL-4, IL-6, and IL-10 are produced by neonates and children than by adults [20].
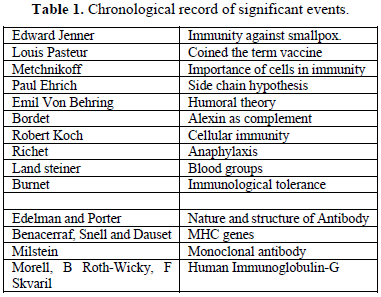
CHRONOLOGICAL RECORD OF SIGNIFICANT EVENTS (TABLE 1)
IMMUNITY
Immunity is concerned with resistance to infection. This is carried out by the process of recognition and disposal of non-self or foreign material that enters the body.
The non-self is usually the life-threatening infectious microorganisms but sometimes it may be tissue grafts taken from other individuals such as the kidney or a piece of skin.
Innate immunity
Innate Immunity is a form of nonspecific host defense against invading bacteria. It is natural or “innate” to the host, depending, in part, on genetics.
Adaptive immunity
Adaptive immunity also called acquired immunity. It is mediated by either B cells (antibody) or T cells (cell-mediated immunity). As core function it recognizes antigenic molecules where, antigens can be “foreign” or “self” and against that cytokines (messengers) are produced. It generally, takes 7-10 days to mobilize on first encounter. It mobilizes much faster on second encounter (memory). They use antigen recognition molecules, antibodies on B cells (BCRs), T cell recognition (TCR) on T cells. Major histo-compatibility antigens (MHC on antigen-presenting cells). Adaptive immunity can be active or passive. The B or T cell encounters the antigen for which it is specific. Reaction with the antigen causes cytokines to be produced.
Cytokines affect other cells AND the cell which produced the cytokine. The cell proliferates into a clone of cells all with the same specificity as the original cell. Thus, the response to the antigen is augmented.
Active immunity
The immunity which results from exposure to an antigen results in natural infection, Vaccination, Passive immunity. Immune components from an exposed individual are transferred to and individual without immunity. Usually antibody. Occasionally cellular Cytokines.
Cytokines include chemokines, interferons, interleukins, lymphokines, and tumor necrosis factors, but generally not hormones or growth factors (despite some overlap in the terminology).
Cytokines are produced by a broad range of cells, including immune cells like macrophages, B lymphocytes, T lymphocytes and mast cells, as well as endothelial cells fibroblasts, and various stromal cells; a given cytokine may be produced by more than one type of cell [21,22].
Cytokines are a variety of soluble proteins secreted by monocytes, lymphocytes and other cells exert profound effects on lymphocyte proliferation and terminal differentiation. These soluble proteins include monokine produced by monocytes, interleukins produced by leukocytes (lymphocytes) lymphokines produced by T-lymphocytes.
These biologically active substances are collectively known as cytokines. They are not specific for antigens.
SUPER ANTIGENS
Superantigens (SAgs) are a class of antigens that result in excessive activation of the immune system. Specifically, it causes non-specific activation of T-cells resulting in polyclonal T cell activation and massive cytokine release [23].
THE T-CELL RESPONSE
The T-Cells originate in the bone marrow and migrate to the thymus for differentiation. Those that recognize self are destroyed. Those that survive are mature but still to be activated.
T-cells have specific TCRs on the surface with binding sites extending to the outside. The two major types of T cells are helper T(CD4+) and cytotoxic (CD8+) T-cells. The major roles of T cells in the immune responses are; Recognition of peptide epitopes presented by MHC molecules on cell surfaces. This is followed by activation and clonal expansion of T-cells in the case of epitopes associated with class 11 MHC molecules. Production of cytokines that acts as intercellular signals and mediate the activation and modulation of various aspects of the immune response and of nonspecific host defenses.
CD4+HELPER T LYMPHOCYTES
Helper T cells (Th cells) are stimulated by antigen in the context of MHC class 11 presentation and are further marked by the presence of the CD4 cell surface antigen.
CD8+ CYTOTOXIC T-LYMPHOCYTES
CD8+Cytotoxic T lymphocytes are a second class of effectors T-cells.
They are lethal to cells expressing the epitope against which they are directed when the epitope is presented by class 1 MHC molecules. They too have specific epitope recognition sites, but they are characterized by the CD8 cell surface marker; thus, they are referred to CD8 + cytotoxic T cells.
The destruction of the virally infected cell is accomplished through a complement like action mediated by perforins, which also facilitate entry into the cell of enzymes (granules) that activate apoptosis [24].
MACROPHAGES
Macrophages are the large eaters. These are the long-lived phagocytes. These cells move over alveolar surfaces, scavenger dust particles, microorganisms and other debris.
The attachment of antigens to macrophage is specific. All the macrophages have specific receptors for C3 component of complement as well as Fc component of antibody. The C3 receptor promotes the adherence of antigen to macrophage by way of opsonization antigen by the complement whereas the Fc receptors help in binding within the antibody there by promoting the phagocytosis of antigen antibody complex. When the antigen adheres with lymphocyte processing, receptors for the antigen, recognition takes place and thus the lymphocytes are induced to produce immunity.
The macrophages that present antigens to T-helper cells (Th) should have MHC determinant of class 11 on the surface whereas macrophage that present antigens to T-cytotoxic cells (Tc) cells should have the MHC determinant, it cannot cooperate and thus antigen presentation cannot occur, which is known as MHC restriction Macrophages are important secretary cells producing and secreting a number of substances such as components of complement system, hydrolytic enzymes, toxic forms of oxygen and the monoclines. Themonokines have regulatory effects on lymphocyte function. Each cell type expresses characteristic surface molecules (CD3, CD4, and CD8.) These cells scavenge the dust particles, microorganisms and other debris.
The primary function of macrophage is phagocytosis. The macrophages, by their property of amoeboid movement, put forth pseudopodia which help in engulfing any solid particle such as the invading microorganisms. The attachment of antigens to macrophage is specific. All the macrophages have surface receptors for C3 component of complement as well as for Fc component of antibody. Phagocytosis of virus and virus-infected cells; Killing of virus-infected cells; and Production of antiviral molecules: TNFa, nitric oxide, and IFN-a.
NEUTROPHILS
Neutrophils only migrate into tissue if there is inflammation.
Monocytes cruise normal tissue When macrophages encounter a signal (infection), they recruit neutrophils to the site. Example, mediate selectins and adhesions which cause neutrophils to stop and migrate into tissue. The term 'left shift' indicates that the neutrophils present in the blood are at a slightly earlier stage of maturation than usual. Inflammatory signaling Mediators produced by macrophages and neutrophils are Prostaglandins.
Lipid derivatives of arachidonic acid they Inhibit platelet aggregation, increase vascular permeability, induce smooth muscle contraction Leukotrienes Lipid derivative of arachidonic acid slow reactive substance of anaphylaxis - SRS-A - But long-term inflammation (chronic) is controlled by macrophages - If not cleared, chronic inflammation turns into a granuloma.
MAST CELLS AND BASOPHILS CELLS
These concentrated within the respiratory and gastrointestinal tract, and within the deep layers of skin. Influenced by TH2, and IL-13 and IL-4 Reside in sub-mucosa, skin, connective tissue Numbers in tissue increase during worm infection IgE binds to Fc receptors on the surface of mast cells Binds to IgE Even though Ig is not bound to antigen Receptor is FceRI One
mast cell has multiple specificities Cross-linking of IgE molecules à activation (Mast cells also activated by C3a, C5 and certain drugs, STAY TUNED) Activation à degranulation and synthesis of mediators Mast cell proteolytic enzymes = tryptase and chymotrypsin These enzymes Increase mucus production - Increase smooth muscle contraction .Cleave and activate complement components and kinin components à inflammation Histamine (à pruritus) .Smooth muscle contraction - Increased vascular permeability .Chemotactic for white cells Cytokines to promote and extend inflammation .TNF àlfa enhanced diapedesis à inflammation - IL-4 stimulates TH2 responses -IL-3 and IL-5 stimulates eosinophil production and activation.
EOSINOPHILS
These cells contain Blobbed Nucleus These are granulocytes. Phagocytic. Granules contain hydrolytic enzymes.
Primary defense against helminth infections (MBP) Eosinophils contains Toxic substances in granules. Derived from the same precursor as PMNs. Influenced by IL-3 and IL-5 (secreted by TH2 cells) Leukotriene are produced by mast cells. Provokes bronchospasm Cationic protein à damages worm plus damages worms nervous system (a neurotoxin).
NEUTRALIZING ANTIBODIES
Immunoglobulin Substances that can produce a specific immune response when introduced into the tissues are antibodies (Ab), Plasma cells & White blood cells. Immunoglobulin (Igs), also known as antibodies, is glycoprotein molecules produced by plasma cells (White blood cells (WBCs).
IgG
It is formed by 2 light chains (K or? type) and 2 heavy chains (? type). Predominant class of immunoglobulins and 70% of total Igs in human serum. Normal serum concentration 8-16 mg/ml. IgG is of greatest amount in internal body fluid and is produced particularly during secondary immune response. IgG has half-life for 25 years. IgG has 4 subclasses IgG1, IgG2, IgG3 & IgG4. I gG is the only Ig that crosses the human placenta and thus often provides protection to the newborn for about 6-9 months. By binding to bacteria, IgG is poisoned thereby enhancing their phagocytosis and elimination. This process is known as “Opsonization”.
IgA
IgA is found only to a small extent in serum (Serum IgA) but predominantly resent in serous and mucus secretions of respiratory, GI and Urogenital tracts. Ig A is the 2nd in abundance among the immunoglobulin (About 10-15%) next to IgG.
IgA has 2 sub classes IgA1 and IgA2. IgA structure is very similar to IgG molecules. Serum IgA It has 4 polypeptide chains, 2 light and 2 heavies. The light chains are either K type or? type. The heavy chain is of a-type. The a-chains of IgA differ from? (gamma) chain of IgG in having greater carbohydrate and amino acid sequence. The normal serum level of IgA is 0.6-4.2 mg/dl. It has a half-life of 6-8 days. Serum IgA has more IgA1 than IgA2. Activate alternative complement pathways. Neutralizes local toxicities, promote phagocytosis. Activated bacteriolytic activity. IgA is found to produce immunity against Tapeworms. A found in colostrum.
IgM
It is the Largest immunoglobulins- referred as “Macroglobulin”.
They are polymers of usually 5 molecules (Pen tamers) each with 4 polypeptide chains. Serum IgM is very low. No sub classes in IgM. Earliest Igs synthesized in foetus-5th month of fetal life. IgM is the most primitive type of Ig. First antibody that appears primary immune response. It is a useful indicator of recent infection. IgM will not cross the placenta. Human fetus can synthesize IgM. IgM detection in the newborn is a useful indicator of recent infection. Useful indicator of intrauterine infections, such as congenital syphilis, rubella, toxoplasmosis. Most natural Abs of ABO blood groups (Anti “A” and anti “B” IgM class. Also helps in “Opsonization”, complement fixation, agglutination & cytolysis. Primarily localized in blood and thus protects against blood, serum, infections.
IgD
IgD has 2 light chain of Kappa (K” Useful or Lambda. IgD is larger than IgG. IgD limited in blood serum. Half-life of IgD is 2-3 days, IgD has 2 subclasses-IgD1 and IgD2. It is found associated with the surface of B-Lymphocytes. IgD is found associated with the surface of B-lymphocytes to act as an antigen receptor.
IgE Two light and 2 heavy chains. Light chains are K or Lambda type. Heavy chains are of e (Epsilon) type. Very low levels of IgE in plasma. But persons with allergies contain 50-100 times higher. High levels are also noted in helminths infections.
It has special attraction to mast cells and basophils containing K as “Skin sensitive antibody”. Originally called “REAGIN”. Clumping of particulate Ags. Igs combine with soluble Ags such as tetanus toxin and the toxin are made inactive Rupture the cell membrane & lysis of RBC. Making Ag more susceptible for phagocytosis Attach tissue cells & responsible for various hypersensitivity reactions in human being Research on Bacterial escape mechanisms Various escape mechanisms are employed by bacteria to elude immune reaction of the host. The bacteria causing pneumonia, meningitis, anthrax and gonorrhea are encapsulated. The capsules are resistant to phagocytic action, but can be punctured by antibodies and complement.
There are some phagocytic bacteria which are devoid of a capsule but have highly resistant cell walls. These bacteria produce chemicals that block the binding of the phagocytes, ultimately killing them (e.g., Bacteria causing tetanus, cholera and diphtheria) some infections cause inflammatory reactions at the infected site due to the activity of cytotoxic and delayed hypersensitivity T-Cells, resulting in tissue destruction and scarring. This is indicated in Tuberculosis and Leprosy [24].
RESEARCH ON INFLAMMATORY RESPONSE
The only thing certain is uncertainty. The change is life’s one constant. Tissue injury such as that following the establishment and multiplication of microorganisms, call forth an inflammatory response. This begins with local arterioles and capillaries, from which plasma escapes.
Edema fluid accumulates in the area of injury, and fibrin forms a network and occludes the lymphatic channels, tending to limit the spread of organisms. Polymorph nuclear leukocytes in the capillaries stick to the walls, and then migrate out of the capillaries towards the irritant. This migration is stimulated by substances from the inflammatory exudate (chemotaxis) The phagocytes engulf the microorganisms and intracellular digestion begins.
Soon the pH of the inflamed area becomes more acid, and the cellular proteases tend to induce lyses of the leukocytes. Large mononuclear macrophages arrive on the site and, in turn, engulf leukocyte debris as well as microorganisms and pave the way for resolution of the local inflammatory process.
IMMUNITY IN BACILLUS CEREUS
Guinea pigs are highly susceptible but rats are resistant to Anthrax infection. This fact can be attributed to variety of defense mechanisms, Leucocyte activity, body temperature and bactericidal action on blood.
THE INNATE IMMUNITY TO S. AUREUS
It is multifaceted and tissue specific. Decades of research on staphylococcal pathogenesis have elucidated important roles for key PRRs, such as TLR2 and NOD2, as well as for specific cytokine signaling pathways, such as IL-1. The roles of tissue-resident cells in these signaling processes are beginning to be explored and will be facilitated by new mammalian genetic tools.
Understanding how innate immune responses impact tissue homeostasis is a critical future direction, given that tissue pathologic condition is a significant driver of morbidity, mortality, and treatment failure [25].
IMMUNITY IN GROUP B STREPTOCOCCUS
Antibody protective against GBS disease, but as with group A Streptococcal M protein, the antibody must be specific to the infecting type of GBS. Fortunately, there are only nine types, and type 111 produces the majority of cases in the first week of life. Antibody is acquired by GBS infection, and specific IgG may be transmitted transplacentally to the fetus, providing protection in the perinatal period.
In presence of type specific antibody, classical pathway C3b deposition, phagocytic recognition, and killing proceed normally.
IMMUNITY IN STREPTOCOCCUS PNEUMONIAE (PNEUMOCOCCUS)
Since the phagocytic capsule is the principal virulence factor of the pneumococcus, the principal protective antibody is antibody specific for the capsular polysaccharide. This antibody exerts strong opsonic effect so that the capsulate pneumococcus is easily phagocytosed. Production of anticapsular antibody in the course of infection is probably the main factor bringing about recovery.
IMMUNITY IN BORDETELLA PERTUSSIS
Studies in murine models have shown that innate immune mechanisms involving dendritic cells, macrophages, neutrophils, natural killer cells, and antimicrobial peptides help to control the infection, while cellular immunity mediated by T-helper type 1 (Th1) and Th17 cells.
IMMUNITY TO HAEMOPHILUS INFLUENZA
Haemophilus influenza is the most common cause of bacterial meningitis in children from 5 months to 5 years of age.
By age 3-5 years many children have anti PRP antibodies that promote complement - dependent phagocytosis. Injection of PRP into children over 2 years of age induces the same antibodies, but children under age 2-years, it is poorly immunogenic. Immunity in Corynebacterium diphtheriae Antitoxic immunity to Corynebacterium diphtheriae may be active or passive. Resistance to the disease depends largely on availability of specific neutralizing anti-toxin in the bloodstream and the tissues. It is generally true that diphtheria occurs only in persons who possess no antitoxins.
IMMUNITY IN MYCOBACTERIUM TUBERCULOSIS
Innate immune cells in the lungs, primarily macrophages, dendritic cells, monocytes, and neutrophils, readily phagocytose M. tuberculosis and are the earliest defenders against the pathogen. In this regard, the production of IFN-y, which can activate infected myeloid cells and inhibit bacterial replication, is a well-known antimycobacterial contribution by adaptive immune cells such as CD4 and CD8 T-cells [26].
TNF-a is a critical pro-inflammatory cytokine in immunity against M. tuberculosis infection and can be secreted by a number of innate and adaptive immune cells.
The importance of TNF-a in antimycobacterial immunity is clearly demonstrated by heightened susceptibility of TNF-a antibody-depleted animals or in animals lacking TNF receptor signaling following M. tuberculosis infection [27].
IMMUNITY IN MYCOBACTERIUM LEPRAE
Mycobacterium leprae antigens have been demonstrated in the nerves and skin of patients experiencing type 1 reactions. The antigens were localized to Schwann cells and macrophages. Schwann cells have been shown to express TLR2 [28,29].
Mycobacterium leprae infection may lead to the expression of major histocompatibility complex (MHC) II on the surface of the cells, and this may give rise to antigen presentation, which triggers CD4 lymphocyte killing of the cell [30].
Immunohistochemistry studies show greater TNF staining in the skin and nerves during type 1 reaction compared with non-reactional controls [31].
There is a shift towards increased Th1 immunity, and lesions in reaction express the pro-inflammatory cytokines IFN-y, IL-12 and the oxygen free radical producer inducible nitric oxide synthase [32].
IMMUNITY IN ENTEROBACTERIACEAE
(Klebsiella pneumoniae, Salmonella typhi, Shigella schottmiilleri).
Specific antibodies develop in systemic infections. Antibodies against the core glycolipid of Enterobacteriaceae are associated with protection against the hemodynamic sequelae of bacteremia due to gram negative rods and also reduce fever response and augment intravascular clearance of certain organisms.
Infection with Salmonella typhi, usually confers a certain degree of immunity. Reinfection may occur but is often milder than the first infection. Secretory IgA antibodies may prevent attachment of Salmonellae to intestinal epithelium. Injection of killed Shigella stimulates production of antibodies in serum but fails to protect human against infection [33].
IMMUNITY IN VIBRIO CHOLERAE
In experimental animals, specific IgA antibodies occur in the lumen of the intestine.
Similar antibodies in serum develop after infection but last only a few months. The relative role of vibriocidal antitoxic antibodies in the circulation and in the gut are not established.
IMMUNITY IN INTRACELLULAR BACTERIA (CHLAMYDIA TRACHOMATIS, AND RICKETTSIA RICKETTSII)
Many intracellular bacteria such as chlamydia and rickettsia dwell within nonprofessional APCs, which do not express MHC class II constitutively at high levels and lack the expression of co-stimulatory molecules [34].
Intracellular bacteria can cause detrimental inflammation and tissue damage, for example, as a result of IFN-y- and TNF-a-mediated Th1 responses. Under normal circumstances, control mechanisms are in place to limit immunopathology. The main cytokines that limit inflammation and control IFN-y production are IL-10 and TGF-ß [34].
The first and most important immune response to Chlamydia infection is a local one, whereby immune cells such as leukocytes are recruited to the site of infections, and subsequently secrete pro-inflammatory cytokines and chemokines such as interferon gamma [35].
T- and B cell-deficient mice are highly susceptible to rickettsial infections cells can contribute to protection by the production of antibodies. Here, it seems that antibodies that are produced in the absence of CD4+ T cell help as it is the case in the early phase of infection are less protective than antibodies generated with T cell help [36,37].
ADVANCED RESEARCH IN IMMUNOLOGY
The development of monoclonal antibodies is leading to the better recognition and quantization of the different lymphocyte subsets and how they can be modified to produce selective immune-suppression.
Experience with new immunosuppressive drugs, such as cyclosporin, is increasing. The next few years will see the HLA association with disease being largely rewritten bas more precise and detailed analysis of the HLA region on chromosome 6 is achieved, again with the help of monoclonal antibodies. Exciting new developments can be expected from the further study of the role of the HLA system in determining the individual's immune responses.
With improved genetic and immunological markers for disease susceptibility, it should be possible with in the not-too-distant future to initiate effective immunosuppressive therapy at an early stage of disabling immunological disorders [38].
IMMUNOGENICITY AND REACTOGENICITY
These are sustained by inflammatory reactions (innate immunity). The difference is usually at the level of inflammatory reactions involved in both cases. A particular level of inflammatory reactions is required to sustain a good adaptive immune response. Excessive inflammatory reactions, on the contrary, impair the same adaptive immune response by causing serious inflammatory and/or oxidative conditions in certain cases.
CONCLUSION
Prepare and prevent rather than repair and repent Humans evolved with a unique immune system consisting of innate and adaptive immunity. Cell-mediated immunity provides protection using different cells such as white blood cells (WBC), Macrophage, Phagocytes, Dendritic cells and T cells (CD4 and CD8), etc. Humoral immunity is a function of B cells, memory cells and antibodies.
The immune response in human and other higher vertebrates consist of activity of different cells and immune players for identification of the foreign substance, presentation to the immune system followed by killing/removal. There is growing research evidence demonstrating that the animal-based bioactive molecules are being tested and used clinically for various purposes such as antiphrastic, antimicrobial, anti-inflammatory, clot buster and antiviral etc. The clot-dissolving enzyme, peptides, and proteolytic enzyme are classic examples studied tremendously in past from various sources [40].
No Files Found
Share Your Publication :