-
Publish Your Research/Review Articles in our High Quality Journal for just USD $99*+Taxes( *T&C Apply)
Offer Ends On
HPT Ammon*
Corresponding Author: HPT Ammon, Department of Pharmacology, Toxicology and Clinical Pharmacy Institute of Pharmaceutical Sciences, University of Tuebingen, Auf der Morgenstelle 872076 Tubingen, Germany.
Received: August 21, 2023 ; Revised: September 11, 2023 ; Accepted: September 14, 2023 ; Available Online: September 26, 2023
Citation: Ammon HPT. (2023) Inhibition of NFĸB-Activation as a Possible Strategy to Prevent/Treat Diabetes Mellitus? Effects of Boswellic Extracts and Boswellic Acids. J Clin Immunol Res Ther, 2(1): 1-10.
Copyrights: ©2023 Ammon HPT. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Views & Citations
Likes & Shares
NFĸB a possible target for antidiabetic drugs?
Proinflammatory cytokines products of NFĸB activation deriving from immune competent cells, seem to play an important role in diabetes mellitus.
In type 1 diabetes these cells enter into pancreatic islets (insulitis) where they release proinflammatory cytokines which in turn lead to ß-cell death and insulin deficiency.
In type 2 diabetes, which is mainly the result of overweight, apoptosis of fat cells causes inflammation of visceral adipose tissue. Infiltrated immune competent cells, here, also release proinflammatory cytokines, which, after entering circulation, cause insulin resistance in peripheral tissues and the complications of this disease.
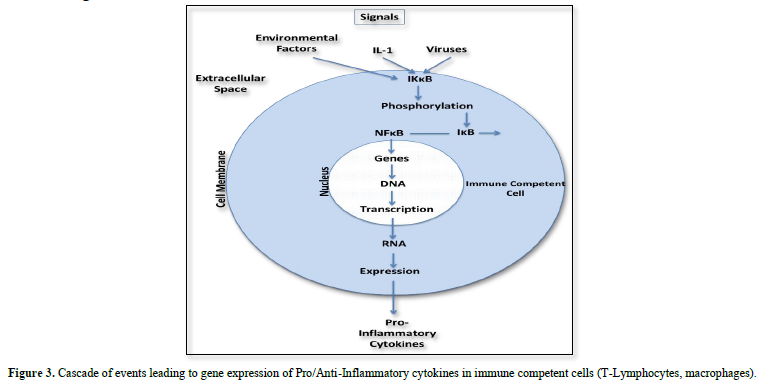
Expression of proinflammatory cytokines follows a cascade of events initiated through activation of the nuclear transcription factor ĸB (NFĸB). In the resting state NFĸB is coupled to the inhibitory protein ĸB (IĸB) as inactive NFĸB-IĸB complex. Its activation results from phosphorylation of this complex induced through intracellular protein kinase B (IKK). In this case the phosphorylation dissociates IĸB from the inactive IĸB-NFĸB complex and NFĸB becomes active to express proinflammatory cytokines.
Having this in mind, drugs inhibiting of NFĸB activation should be considered to prevent/treat type 1 and type 2 diabetes mellitus. That such a strategy might be possible arises from recent studies with boswellic acids, ingredients of the gum resin of Boswellia species. Boswellic acids have been shown to inhibit expression of proinflammatory cytokines by preventing activation of NFĸB via inhibition of the phosphorylation of the inactive NFĸB-IĸB complex.
Using animal models with autoimmune diabetes including the multiple low doses of streptozotocin (MLSTZ) and the non-obese diabetic mouse (NOD mouse) it was shown that boswellic acids (KBA and AKBA) prevented insulitis via inhibition of infiltration of CD3 lymphocytes into pancreatic islets and inhibited NFĸB activation, expression of proinflammatory cytokines and increase of blood glucose.
In animal models with type 2 diabetes where overweight was induced through high fat diets boswellic acids containing extracts from Boswellia resin also inhibited expression of proinflammatory cytokines in immune competent cells. This was associated with improvement of metabolic parameters including glucose and lipids. Moreover, some clinical studies have shown that administration of boswellic acids containing preparation also inhibit hyperglycemia and hyperlipidemia in patients with type 2 diabetes.
Conclusion: The presented evidence using boswellic acids and extracts from the resin of Boswellia species as tools to inhibit of NFĸB activation suggests, that it may be possible, that inhibition of NFĸB activation could be a strategy to prevent/treat type 1 diabetes, LADA and type 2 diabetes. However, the evidence presented here must receive conformation by well-designed clinical studies.
Keywords: Diabetes mellitus, NFĸB, Proinflammatory cytokines, Prevention, Boswellic acids, Inhibition of NFĸB activation
Abbreviations: AKBA: Acetyl-11-Keto-ß-Boswellic Acid; A-ß-BA: Acetyl-ß-Boswellic Acid; AαBA: Acetyl-α-Boswellic Acid; BA: Boswellic Acid; BMI: Body Mass Index; BE: Boswellic Extract; HDL: High Density Lipoprotein; IĸB: Inhibitory Protein Kappa B; IKK: ~ IKKB IKKB: IKB Kinase; IA2-A: Tyrosine Phosphatase A2 Antibody; IFN-γ: Interferon-γ; IL-1, IL-1A, IL-1B, IL-ß, IL-2, IL-6, IL-12: Interleukines; KBA: Keto Boswellic Acid; LADA: Late Onset Auto Immune Diabetes of the Adult; LDL: Low Density Lipoprotein; LPS: Lipopolysaccharide; METS: Metabolic Syndrome; NFkB: Nuclear Transcription Factor Kappa B; NOD: Non-Obese Diabetic; PBMC: Peripher Blood Mononuclear Cell; SGPT: Serum Glytamate Pyrurat Transaminase; SGOT: Serum Glutamatic Oxalacetic Transaminase; STZ: Streptozotocin; TH1, TH2: T-Lymphocytes; TNF-α: Toumor Necrose Factor-α; TLR: Tolreceptor
INTRODUCTION
Diabetes mellitus is a disease where finally insulin deficiency and insulin resistance cause the metabolic disorders including hyperglycemia and hyperlipidemia. Major reasons for this illness are genetical predispositions, environmental factors, viruses and others, leading to inflammatory processes. These are involved in type 1 and type 2 diabetes as well as diabetic complications following insulin deficiency and insulin resistance i.e., vascular diseases and renal failure. There is evidence, that in these cases inflammations are related to over- expression of proinflammatory cytokines.
TYPE 1 DIABETES AND LATE ONSET AUTOIMMUNE DIABETES OF THE ADULT (LADA)
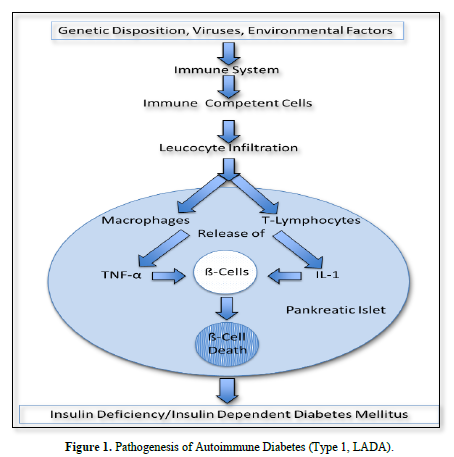
In this case immune competent cells (T-lymphocytes, macrophages) infiltrate into pancreatic islets. There, through activation of NFĸB they release proinflammatory cytokines which cause ß-cell death and insulin deficiency.
In this connection Diaz-Ganete [1] induced type 1 diabetes in mice by the administration of a cytokine cocktail containing IL-1ß, IFN-γ and TNF-α. In this model Ghrelin, which is a peptide that stimulates cell proliferation and inhibits apoptosis in several tissue including pancreas, downregulated the apoptotic actions of the cytokines and restored insulin secretion. In patients with type 1 diabetes Cnop [2] reported an increase in NFĸB, IFN-γ, TNF-α, IL-1 and IL-2 in splenocytes and peripher blood mononuclear cells (PBMCs) (Figure 1).
TYPE 2 DIABETES AND THE METABOLIC SYNDROME (METS)
In type 2 diabetes excess nutrition, obesity, lack of physical activity are the major reasons. There is general agreement that these factors are responsible for the insulin resistance of peripheral tissues. Here, proinflammatory cytokines also seem to play a major role. Moreover, the release of adiponectin is diminished, contributing to decreased insulin sensitivity.
Janochova [3] interpreted an association between obesity, proinflammatory cytokines and insulin resistance as follows:
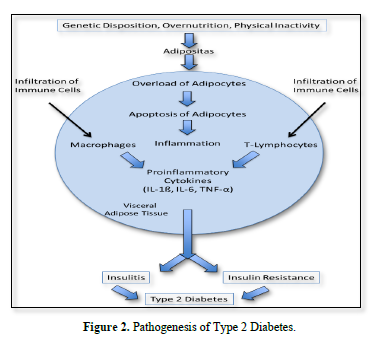
The storage capacity of lipids in adipocytes is limited. Overload leads to increased apoptosis of fat cells that in turn causes infiltration of macrophages particular into the visceral adipose tissue.
These cells produce proinflammatory cytokines while production of adiponectin is decreased. According to Burhans [4] even low-grade adipose tissue inflammation is associated with reduced expression of adiponectin, infiltration of macrophages and other immune cell populations into adipose tissue, there causing increased production of proinflammatory cytokines (IL-1ß, IL-6, TNF-α). Interestingly the visceral adipose tissue is more sensitive to inflammation than the subcutaneous one (Figure 2).
A relationship between proinflammatory cytokines and insulin resistance has been documented in a variety of clinical studies. In this connection, Reinehr [5] reported a relationship between proinflammatory cytokines (TNF-α, IL-1ß, IL-6 and IFN-γ) and insulin resistance and MetS in children. Zhu [6] observed increased IL-6 levels in the aqueous humor of the eyes in patients with insulin resistance and cataract.
In a comparative study in patients with MetS, there was a significant correlation between the serum levels of IL-6 or TNF-α and insulin resistance [7].
In another study with obese type 2 diabetic patients Hetta [8] the authors reported a strong positive correlation between the serum levels of IL-6, TNF-α, body mass index (BMI) and insulin resistance.
On the other hand, Hetta [9] in a clinical study observed, that blockade of the IL-6 receptor by an IL-6 receptor antagonist (tocilizumab) significantly reduced insulin resistance during the time of infusion and increased insulin sensitivity.
While the association between proinflammatory cytokines and insulin resistance in type 2 diabetes is obvious, also chronic inflammation of pancreatic islets seems to play a role. In this connection the nature of islet inflammation and its effect on islet function were studied by Butcher [10] They assayed human islets from organ donors with or without type 2 diabetes. It was found that islets from type 2 diabetic patients displayed higher TNF-α expression than islets from healthy persons. The elevated total islet leucocyte content and proinflammatory mediators correlated with islet dysfunction, suggesting that insulitis also occurs during the development of type 2 diabetes. As discussed above, expression of proinflammatory cytokines is the consequence of NFĸB activation. In this connection Andreasen [11] reported that patients with type 2 diabetes exhibited a more pronounced NFĸB binding to DNA in muscular tissue than non-diabetic patients when stimulated with an intravenous bolus of E-coli LPS. This was associated with dysregulated glucose uptake.
DIABETIC COMPLICATIONS AND INFLAMMATORY SIGNALING
The signaling action of proinflammatory cytokines, as discussed above, holds not only for the very early steps of diabetes, i.e., insulitis and insulin resistance but in addition plays a role in the pathophysiology of the complications following hyperglycemia and hyperlipidemia. In case of the diabetic nephropathia this has been intensively discussed by Rayeo-Mateos et al. (2020) [12]. Here, the use of inhibitors of IL-1A and IL-20 in diabetic animals exhibited amelioration in renal function.
As recently reported activation of NFĸB signaling is induced via so called Toll-like receptors (TLRs) [8,13-15]. In this case in a mouse model with advanced diabetic nephropathia administration of the TLR4 antagonist CRX 5266 significantly improved renal function. On the other hand, selective blockade of IĸB kinase (IKK) had also renoprotective effects in experimental models by reducing NFĸB activation [16-20].
Conclusion-Outlook
The discussed evidences clearly suggest that initiation of type 1 and type 2 diabetes as well as theire complications are related to overexpression of proinflammatory cytokines following the activation of NFĸB.
The present pharmacological strategies to treat diabetes mellitus consist in stimulation of insulin secretion, substitution of insulin, delay of glucose absorption, increase of glucose excretion, glucose utilization, insulin sensitivity as well as inhibition of glucose production in the liver. At present there exists no strategy to interrupt any cascade of events leading to islet destruction, insulin resistance and diabetic complications.
Considering the discussed single steps in signaling leading to the disease it should be possible to develop drugs interrupting overexpression of proinflammatory cytokines at their starting point which is inactivation of NFĸB (Figure 3).
In 2013 there appeared a report indicating that celastrol, a pentacyclic triterpene, isolated from Triperygium uniflora and Celastrus regelii root extracts, which are used as antiphlogistic remedies in traditional Chinese medicine showed beneficial effects on insulin resistance, body weight, renal injury and proinflammatory cytokine levels through NFĸB-B inhibition [21]. Pentacyclic triterpenes are also constituents of the gum resin of Boswellia species used for thousands of years in the traditional Ayurvedic Medicine in India for the treatment of various diseases especially with inflammatory background.
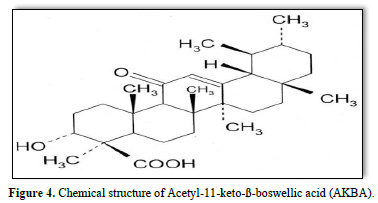
In Europe the gum resin of Boswellia species-its pharmaceutical name is Olibanum Indicum, now part of the (European Pharmacopoeia 8th edition, 2016) has also been used for thousands of years for the treatment of various diseases, including inflammatory disorders [22]. Thus far, more than 216 constituents have been identified in this resin. Among these are boswellic acids, which belong to the class of pentacyclic triterpenes. At presents, most scientific interest is attributed to 11-keto-ß-boswellic acid and 0-acetyl-11-keto-ß-boswellic acid (Figure 4) and their anti-inflammatory properties.
RESIN OF BOSWELLIA SPECIES AND BOSWELLIC ACIDS AS INHIBITORS OF NFĸB ACTIVATION AND EXPRESSION OF PRO-INFLAMMATORY CYTOKINES
Anti-inflammatory actions
In 1986, Singh and Atal firsts reported that an alcoholic extract from the gum resin of Boswellia serrata-in India called salai guggal-inhibited edema production induced by carrageenan injection into rat paws, which is an acknowledged pharmacological model to test the anti-inflammatory effects of drugs. These observations initiated various preclinical studies concerning the mechanism of the anti-inflammatory actions of boswellic extracts (BEs) and boswellic acids (BAs) as well as related clinical trials in patients suffering from chronic inflammatory diseases with autoimmune character.
Immune system
In this connection many studies dealt with the question whether or not extracts from the gum resin of Boswellia species and pharmacological active compounds might interfere with factors of the immune system especially proinflammatory cytokines and their expression through activation of NFĸB.
In 2005 Chorvier et al. [23] reported that an extract of sesame oil from the resin of Boswellia carterii produced a dose-dependent inhibition of IL-2 and IFN-γ in murine splenocytes. Gayathri [24] observed that a crude extract from Boswellia serrata inhibited TNF-α, IL-1 and IL-6 in cultured peripheral blood mononuclear cells (PBMCs). Observations of TH1, /TH2 lymphocyte cytokines also revealed the downregulation of IFN-γ and interleukin-12 (IL-12) following treatment with a methanolic crude extract in PBMCs.
Employing an acetone extract from Boswellia carterii in an adjuvant arthritis model, Fan [25] observed a significant decrease in the arthritis scores that was associated with the suppression of local tissue IL-1ß and TNF-α.
In a recent study where pancreatitis was induced in mice by caerulein, acetyl-α-boswellic and acetyl-ß-boswellic acid protected the disease and reduced expression of proinflammatory cytokines [25].
As discussed, the expression of proinflammatory cytokines, including IL-1, IL-2, IL-6, IFN-γ and TNF-α, is tightly regulated by the transcriptional factor NFĸB. In monocytes, acetyl-α-boswellic acid (AαBA) and AKBA downregulated TNF-α expression. Both also inhibited NFĸB signaling [26]. In 2006, Takada [27] showed that AKBA potentiated apoptosis, inhibited invasion and abolished osteoclast genesis by suppressing NFĸB-regulated gene expression in mice, and Cuaz-Pérolin [28] reported that in an isolated cell system of lipopolysaccharide (LPS)-challenged ApoE-/-mice, AKBA inhibited the activity of NFĸB. Such effects have also been reported to be true for other ingredients of Boswellia resins [29].
In a study in rats where arthritis was induced by collagen, 21 days treatment with 40 and 60 mg/kg of a boswellic extract preparation (Boswellia super®F) containing 30 % acetyl-11-keto-boswellic acids, showed significant inhibition of inflammation (TNF- α and IL-b secretion) and downregulated RNA levels of TNF-α, IL-6 and IL-1ß in macrophages. The extract also reduced the levels of phosphrylated NFkB suggesting an anti-inflammatory activity mediated by blocking this key signal transduction pathway Majeed [30].
When mice with severe psoriasiform lesions were treated with AKBA, NFĸB signaling and subsequent NFĸB-dependent cytokine production, as shown by TNF-α production of macrophages, were profoundly suppressed. This was associated with the improvement in the psoriasis disease activity score [25,31].
Using a model of lipopolysaccharide-mediated TNF-α induction in monocytes, Syrovets [26] postulated that inhibition of NFĸB signaling by boswellic acids is due to their inhibitory action of the phosphorylation of the IKB-NFĸB complex by IKKB (Figure 5).
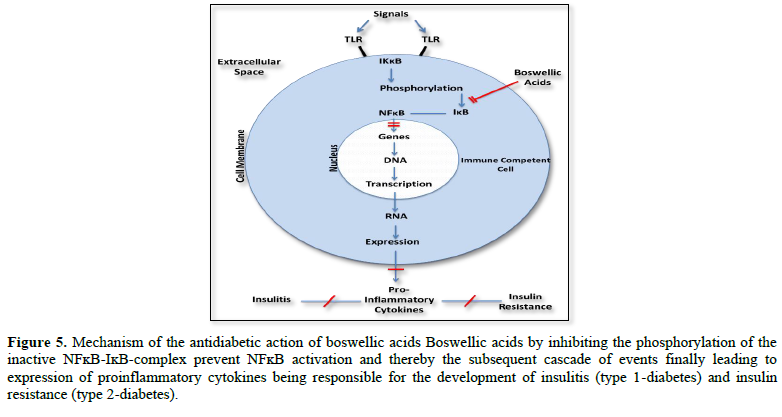
In an Alzheimer model in mice acetyl-11-keto-ß-boswellic acid ameliorated cognitive deficits. Among others this was associated by declining phosphorylation of the inhibitor of nuclear factor-kappa B alpha (IĸBα). Collectively, authors findings provide evidence that AKBA protects neurons among others via nuclear factor-kappa B signaling pathways [32].
Concluding remark, the data discussed thus far demonstrate convincingly that boswellic extracts as well as some boswellic acids, including KBA and AKBA, target the immune system by decreasing the expression of proinflammatory cytokines through inactivation of NFĸB signaling.
BOSWELLIC EXTRACTS AND BOSWELLIC ACIDS AS AN EXAMPLE TO INHIBIT NFĸB ACTIVATION IN DIABETES MELLITES
The discussed effects of boswellic acids on immune system in mind some authors used boswellic acids as an example to study whether or not these inhibitors of NFĸB activation might be useful in prevention and/or treatment of diabetes mellitus.
AUTOIMMUNE DIABETES (TYPE 1 AND LADA), PRECLINICAL STUDIES
In this connection, some studies using boswellic acids have been performed in animal models of autoimmune diabetes, including the administration of multiple low doses of streptozotocin (MLD-STZ) in mice and the non-obese diabetic (NOD) mouse, as recently summarized by Ammon 2019 [33].
The MLD-STZ model: Using this model, Shehata [34] administered 50 mg/kg of streptozotocin (STZ) for five days i.p. into male mice. Five days after the last injection of STZ, the authors observed infiltration of CD3 lymphocytes into pancreatic islets, an increase in proinflammatory cytokines, i.e., IL-1A, IL-1B, IL-2, IL-6, TNF-α and IFN-γ in the blood, some periinsular apoptotic cells and a small but significant elevation in blood glucose that increased further in the subsequent 25 days.
EFFECTS OF AN EXTRACT FROM GUM RESIN OF BOSWELLIA SERRATA
When in the study of Shehata [34] in addition to STZ the animals for 10 days received i.e., 150 mg/kg of a boswellic extract containing 4.66% AKBA and 5.48% KBA, 10 days after starting the experiments, no increase of proinflammatory cytokines in the blood was observed. Furthermore, no infiltration of CD3 lymphocytes into pancreatic islets and the appearance of periinsular apoptotic cells could be detected. As far as blood glucose levels are concerned, no significant increase was detectable even after week four. The data suggest that the extract must have interrupted the STZ signal at a very early step in the cascade of events leading to the expression of proinflammatory cytokines possibly at the level of NFĸB. This interpretation is in line with Cuaz-Pérolin [28] reporting the inhibition of NF-ĸB by boswellic acids.
Effects of Boswellic acids: Of special interest among boswellic acids are ß-boswellic acids, which include ß-boswellic acid (ß-BA), acetyl-ß-boswellic acid (A-ß-BA), 11-keto-ß-boswellic acid (KBA) and 0-acetyl-11-keto-ß-boswellic acid (AKBA).
Using the MLD-STZ [36] model only the keto-forms of boswellic acids i.e., KBA (7.5 mg/kg i.e.) and AKBA (15 mg/kg i.e.), inhibited infiltration of CD3 lymphocytes into pancreatic islets, appearance of peri-insular apoptotic cells and significantly reduced the STZ-mediated increases of proinflammatory cytokines (IL-1A, IL-1B, IL-2, IL-6, IFN-γ, TNF-α) in the blood. Regarding the blood glucose levels, KBA was most effective in inhibiting STZ-mediated hyperglycemia whereas ß-BA and A-ß-BA showed no effect [37]. From these data it appears that KBA and AKBA play important roles in the Boswellia extract to prevent autoimmune diabetes in this model. However, it is possible that also other ingredients of the resin may exhibit a protective action.
The NOD mouse model: In contrast to the MLD-STZ model where a chemical agent, i.e., STZ, produces a short, but severe and rapid action on immune competent cells, the initiation of autoimmune diabetes in the NOD mouse is quite different and due to a genetic disorder. Here, some female animals develop insulitis, which starts about 4 weeks after birth [35]. Due to the slow progression of the inflammatory process, it takes about 18 weeks until damage of the ß-cells leads to insulin deficiency and consequently to an increase of blood glucose.
Accordingly, in this model in control animals Shehata [38] observed no infiltration of CD3 lymphocytes at week 4, but infiltration of CD3 lymphocytes and appearance of some periinsular apoptotic cells appeared at week 7 after birth within this period there was no increase of blood glucose.
Also, in the NOD mouse activation of NFĸB has been shown to be related to insulitis. Interestingly activated NFĸB was detectable only at low levels over background and did not vary with age. This may explain the very slow development of the disease [36].
Effects of KBA: Since in the MLD-STZ experiments, KBA turned out to be the most effective boswellic acid, in this model, Shehata [38] administered KBA in a dose of 7.5 mg/kg i.p. daily from week 4 to week 7. This treatment caused significant inhibition of CD3 lymphocyte infiltration into pancreatic islets and no appearance of periinsular apoptotic cells. No decrease in the blood glucose could be observed within this period. However, similar to untreated controls the blood glucose sharply increased in weeks 18-20 indicating that after discontinuation of KBA administration in week seven, in contrast to the STZ experiments in the NOD mouse the immune process started again [38].
CLINICAL CASE REPORTS
So far there exist no clinical studies dealing with boswellic acids in patients with autoimmune diabetes. There is only one case report in a LADA-patient showing that oral administration of a Boswellia extract preparations containing 3.6 % KBA and 1.4 % AKBA significantly reduced a marker of autoimmundiabetes i.e., tyrosine phosphatase A2 antibody (IA2 – A) in the serum nearly to normal [39].
A further case report shows similar results. Here, Franic [40] report that in a patient with LADA 9 months treatment with an extract from Boswellia serrata gum resin decreased GAD 65 autoantibodies in the blood by 25 %, a further marker of insulitis.
Type 2 Diabetes
As discussed above, NFĸB activation and the following expression of proinflammatory cytokines play also a role in type 2 diabetes and Mets. Haying this in mind it is logical to study whether or not inhibition of NFĸB activation could be a strategy for prevention/treatment of type 2 diabetes and Mets. In the preclinical studies with type 1 diabetes as an example for drugs, that could inhibit NFĸB activation, boswellic acids have been employed. Unfortunately, this was not the case in the preclinical studies with type 2 diabetes models. In these studies, extracts from the gum resin of Boswellia species with defined contents of boswellic acids were used. In focus were studies examinating the effect of resin preparations of Boswellia species on proinflammatory cytokines and on the consequences of insulin resistance i.e. increased metabolic parameters including blood glucose, HbA1c and lipids.
PRECLINICAL STUDIES WITH BOSWELLIC EXTRACTS
In a study of Gomaa (2018a) [41] where in rats’ obesity was induced by a high-fat diet, the administration of a polyphenol-rich extract from Boswellia gum resin significantly decreased food intake and prevented obesity. This was associated with reduced serum levels of IL-1ß, TNF-α, glucose, triglycerides, LDL-cholesterol and insulin, while the levels of adiponectin and HDL-cholesterol were significantly increased. The results were interpreted to be due to suppression of insulin resistance as a consequence of TNF-α and IL-1ß reduction along with increasing adiponectin levels.
In a second study, Gomaa (2018b) [42] induced type 2 diabetes in rats by a high-fat/high-fructose diet together with a single injection of streptozotocin. The rats received 3 doses of a polyphenol-rich extract of Boswellie serrata gum resin. This treatment reduced hippocampal TNF-α, IL-1ß and IL-6. In addition, the extract alleviated insulin resistance and hyperlipidemia.
CLINICAL STUDIES WITH BOSWELLIC RESIN/EXTRACTS
Again, using boswellic extracts the clinical studies dialed with the question whether or not inhibition of NFĸB activation prevents increases of metabolic parameters in type 2 diabetes.
In a randomized, double-blind, placebo-controlled trial, Azedmehr et al. [43] studied the effect of the gum resin of Boswellia serrata on blood glucose and lipid parameters in 71 patients with type 2 diabetes. All patients were also under treatment with metformin. In addition to metformin the intervention group received 400 mg of the resin orally twice daily for 12 weeks. Compared with the placebo group (metformin only), in the intervention group, there was a significant reduction in fasting blood glucose, HbA1c and serum insulin. Moreover, a decrease of serum cholesterol, LDL and triglycerides was reported. No adverse effects were observed [43].
In another double-blind, randomized and placebo-controlled study Mehrzadi [44] with type 2 diabetics, however, the gum resin of Boswellia serrata was not found to be effective when given orally twice daily at 250 mg for 8 weeks in addition to their routine anti-diabetic treatment. This discrepancy to the study of Azedmehr et al. [43] could be explained by the lower dose of the resin, and shorter duration of treatment.
Unfortunately, due to ethical reasons, both studies were not be performed without an additional anti-diabetic therapy.
Nevertheless, a study of Ahangarpour [45] testing a possible hypolipidemic action of Boswellia serrata gum resin is in line with the data of Gomaa (2018b) [42]. Here, 60 type 2 diabetic patients from both sexes were dedicated to a control and an intervention group (30 subjects per group). Boswellia serrata gum resin at 900 mg daily was administered orally for 6 weeks (as three 300 mg-doses) in the intervention group. The control group did not receive anything (i.e., no placebo). Blood samples were taken at the beginning of the study and after 6 weeks. In this study, the authors observed a significant increase in blood HDL as well as a remarkable decrease in cholesterol, LDL, triglyceride, fructosamine, SGPT and SGOT levels.
Thus, at least the studies of Azedmehr et al. [43] and Ahangarpour [45] showed improvement in type 2 diabetics receiving the gum resin of Boswellia species.
DISCUSSION
Activation of the nuclear transcription factor NFĸB through the action of various factors including genetic predisposition, viruses and others is the first step in a cascade of events finally leading to type 1 and type 2 diabetes. If this is correct, measures to inhibit activation of NFĸB should be a strategy to prevent development of this disease and its complications.
The data derived from experimental animals with autoimmune diabetes clearly show that treatment with inhibitors of NFĸB i.e., boswellic extracts or some boswellic acids prevent infiltration of CD3 lymphocytes into pancreatic islets, expression of proinflammatory cytokines and increase of blood glucose. Moreover, case reports from patients with LADA report reduction of insulitis markers in the blood.
In type 2 and Mets where insulin resistance is also related overexpression of proinflammatory cytokines the use of boswellic extracts in mice with experimental type 2 diabetes not only decreased serum levels of proinflammatory cytokines, but also metabolic parameters including glucose and lipids which must be interpreted to be the consequence of inhibition of NFĸB signaling. This seems to hold not only for experimental animals but also for patients with type 2 diabetes, where administration of resin preparations of Boswellia species reduced blood glucose HbA1c and lipid parameters, being in line with animal experiments.
CONCLUSION
Thus, taking together, in conclusion, inhibition of NFĸB signaling for instance with boswellic acids/boswellic acids containing preparations may be a strategy to prevent/treat type 1 and type 2 diabetes. This strategy may hold also for other drugs inhibiting NFĸB activation. However, further well-designed clinical studies are warranted to support this new possibility to prevent/treat diabetes mellitus.
ACKNOWLEDGEMENT
The author is grateful to Privatdozent Dr.Susanne Ammon-Treiber, Institute of Pharmaceutical Sciences, University of Tuebingen, for providing editorial support to this manuscript and to Johannes Ertelt, Heidelberg Apotheke, Bisingen, for support in the literature supply.
CONFLICTS OF INTEREST STATEMENT
There exists not any conflict of interest.
No Files Found
Share Your Publication :