-
Publish Your Research/Review Articles in our High Quality Journal for just USD $99*+Taxes( *T&C Apply)
Offer Ends On
Boonyakorn Wongsakul*, Nattawadee Monon, Chanon Fangoen and Chonlatip Pipattanaboon
Corresponding Author: Boonyakorn Wongsakul, Department of Animal Diagnosis and Investigation, Queen Saovabha Memorial Institute, The Thai Red Cross Society, 1871 Rama 4 Road, Pathum Wan, Bangkok 10330, Thailand.
Received: June 06, 2023 ; Revised: June 09, 2023 ; Accepted: June 12, 2023 ; Available Online: June 22, 2023
Citation: Wongsakul B, Monon N, Fangoen C & Pipattanaboon C. (2023) Efficiency Evaluation of Commercially Veterinary Rabies Vaccine after the Primary Immunization against Fatality. J Vet Marine Res, 3(1): 1-7.
Copyrights: ©2023 Wongsakul B, Monon N, Fangoen C & Pipattanaboon C. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Views & Citations
Likes & Shares
The Rabies Virus (RBV) is a contagious zoonotic disease with no known treatment, but it can be prevented through immunization in both humans and animals. The key to successful rabies vaccination lies in the availability of high-quality vaccines capable of eliciting an immune response that reaches the adequate protective level set at 0.5 IU/ml by the World Health Organization (WHO). Furthermore, the vaccination should effectively prevent the occurrence of fatalities in vaccinated animals. Two commercially available rabies vaccines from Thailand were assessed in Syrian Hamsters to determine the Rabies Neutralization Antibody (RVNA) levels post-vaccination and to observe the survival rate. Both vaccines were found to induce immune responses that exceeded the protective threshold of 0.5 IU/ml at one month and the first three months after vaccination (P-value = 0.00 and 0.11, respectively, for Vaccine A, and 0.00 and 0.11, respectively, for Vaccine B). At the sixth month after vaccination, both groups of Syrian hamsters exhibited a Geometric Mean Titer (GMT) of RVNA at the levels of 0.378 IU/ml for Vaccine A and 0.096 IU/ml for Vaccine B, resulting in a 90% survival rate. The surviving animals demonstrated a range of Nab levels between 0.003-1.06 IU/ml. This indicates that an immune level of 0.5 IU/ml alone may not provide sufficient protection against infection. However, a threshold of 0.5 IU/ml should still be considered when establishing rabies-free areas.
INTRODUCTION
Rabies, caused by the Lyssa Virus of the Rhabdoviridae family, is a contagious zoonotic disease that currently lacks a viable treatment method. It is responsible for approximately 59,000 deaths annually [1] and leads to significant economic losses of up to 5.5 billion dollars per year [2]. The primary mode of transmission is through saliva, with most infections occurring via bites or contact with infected animals, primarily rabid dogs. However, the disease can be prevented through immunization of both humans and animals. Many countries aspire to achieve rabies-free status, necessitating collaborative efforts involving multiple stakeholders. These efforts typically involve regular vaccination of dogs and humans, public education campaigns to raise awareness, accessibility to high-quality and sufficient vaccine supplies, and ongoing surveillance. By engaging in such cooperative endeavors, communities can work towards becoming rabies-free zones [3]. Additionally, factors beyond the proper administration of the vaccine, such as vaccine quality, play a crucial role. Reports have emerged from Thailand and other developing countries, highlighting issues of substandard vaccine quality [4] and improper storage before administration. Such vaccines fail to elicit the required levels of Rabies Virus Neutralization Antibody (RVNA) to meet standards and effectively prevent the disease. Consequently, disease control efforts within affected communities often falter.
The assessment of vaccine effectiveness is typically based on the detection of Neutralization Antibody (Nab) levels, specifically RVNA for rabies, as per the standards set by the World Health Organization (WHO). This method gauges the level of rabies immunity post-vaccination, influencing virus clearance from the body and nervous system, thereby preventing infection in both humans and animals [5,6]. The Rapid Fluorescent Focus Inhibition Test (RFFIT) represents the widely adopted approach for measuring rabies Nab levels in both humans and animals. This method involves culturing the virus on a cell line and assessing the inhibition of virus proliferation through serum sample analysis. It is considered the gold standard by both the Centers for Disease Control and Prevention (CDC) and the WHO [7-10]. In accordance with WHO recommendations, the minimum Nab threshold of 0.5 IU/ml is deemed adequate for vaccine protection, serving as the standard response to rabies vaccination. However, it has been observed that an immune level of 0.5 IU/ml alone does not offer sufficient protection against infection [8]. Consequently, even levels below 0.5 IU/ml can enable animals to survive rabies infection and reduce fatality rates, potentially leading to reduced vaccination requirements and consequent cost savings in numerous countries.
This experiment demonstrates the efficacy of commercially available vaccines used in Thailand in boosting immunity and preventing death. After the initial vaccination period, when immunity declines below the standard level (0.5 IU/ml), do the animals retain the ability to prevent disease and survive infection-induced mortality? These findings hold significance for future vaccine administration and can contribute to improved stability and efficacy in rabies prevention efforts.
MATERIAL AND METHOD
Animals and sample collection
The animal model used in this study comprised Syrian Hamsters (n=30) obtained from the Laboratory Animal Unit of the Faculty of Medicine, KhonKaen University. The animals were divided into three groups: the control group (control; n=10), brand A vaccine group (Vaccine A; n=10), and brand B vaccine group (Vaccine B; n=10). They were housed in a closed and temperature-controlled room. Blood samples were collected via heart puncture and centrifuged to obtain a minimum volume of 25 μl of serum. Blood collections were performed before vaccination, as well as at 1, 3, and 6 months after vaccination. All procedures were conducted under anesthesia using "Rodent cocktail" [9]. The laboratory animal ethics protocol was approved by the Queen Saovabha Memorial Institute Animal Care and Use Committee (QSMI-ACUC-04-2018).
Vaccination
The vaccines utilized in this trial were two inactivated vaccines commercially available in Thailand: Bayovac-R® (Bayer Thai Co. Ltd., Bangkok, Thailand) and RABISIN® L473278 (Merial [Thailand] Ltd., Bangkok). To induce immunity, both brands of vaccine were administered via intramuscular injection in the gluteus muscles of the experimental hamster groups. The control group received a normal saline solution injection at the same site for placebo purposes.
Virus Challenge
The virus employed in this study was the Thai street dog rabies strain, harvested from diagnosed cases by the Department of Animal Diagnosis and Investigation, QSMI. The virus titer was determined to be 107.023 LD50/mL through intracranial injection in laboratory mice, observing the survival rate after varying dilutions, and calculating using the Reed and Muench method [11]. A volume of 100 microliters of the virus (titer of 106.023) was injected intramuscularly into all three groups. Symptoms and survival rates were observed for 8 months.
Sample identification
Post-vaccination immunity was assessed using the Rapid Fluorescent Focus Inhibition Test (RFFIT) [7], a highly sensitive and specific test for Rabies Virus Neutralizing Antibody (RVNA). The Mouse Neuroblastoma (MNA) cell line was employed, cultured in Minimal Essential Medium (MEM) supplemented with L-glutamine (100X), penicillin (100 IU/ml), streptomycin (100 μg/ml), and gentamicin (10 μg/ml), along with 10% Fetal Bovine Serum (FBS) (Gibco, USA). The cell culture was incubated at 37°C with 5% CO2 for 2-3 days until a confluent monolayer was obtained. Challenge Virus Standard (CVS) was used as the test control. The flasks were incubated at 37°C with 5% CO2. The results were expressed in IU/ml, with a cutoff value of 0.5 considered indicative of adequate rabies vaccination [8]. In cases of deceased hamsters, an autopsy was performed, and a brain sample was obtained for an impression smear and subjected to the Direct Fluorescent Antibody (DFA) test, the gold standard for rabies diagnosis. The test involved fluorescent staining to detect virus particles in the brain sample [12,13].
Data analysis
Nab values were measured in IU/ml and averaged as a geometric mean titer (GMT). The changing Nab levels over time for each vaccine brand were compared using Repeated Measures ANOVA at a confidence level of P=0.05. Monthly Nab levels among Group A, Group B, and the Control group were compared using one-way ANOVA (P<0.05), and Tukey's Multiple Comparison Test was employed to calculate the survival rate after viral challenge. The statistical analysis was performed using SPSS version 25 (IBM Corporation, United States).
RESULTS
Control Group (Placebo)
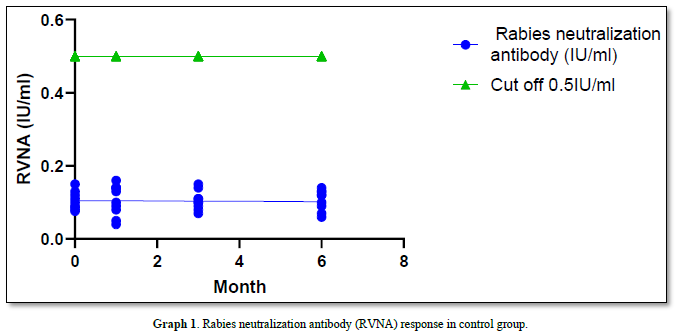
The geometric mean titers (GMT) of Rabies Virus Neutralizing Antibody (RVNA) immunity at 0 months, 1 month, 3 months, and 6 months were 0.100 IU/ml, 0.098 IU/ml, 0.098 IU/ml, and 0.097 IU/ml, respectively. None of these values reached the standard protection level of 0.5 IU/ml, and there was no significant difference in immunity observed between the different months (all P-value = 1.00). The survival rate after challenging the animals with the virus via intramuscular injection was 0% (all samples died). The results of the Direct Fluorescent Antibody (DFA) test were positive for all samples, with deaths occurring on days D12 to D18 (Graph 1).
Group A (RABISIN®)
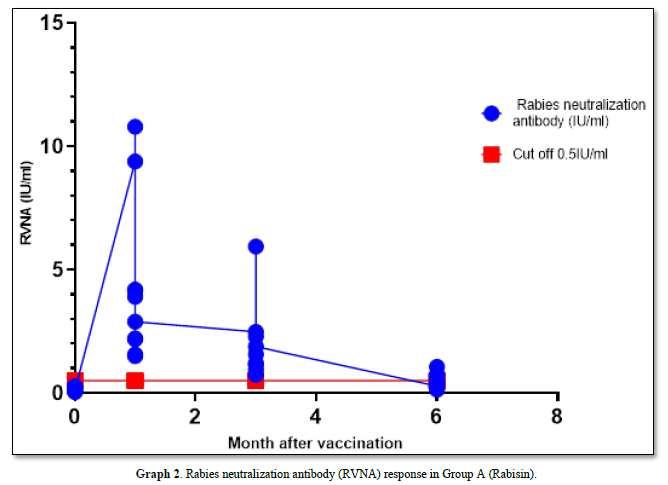
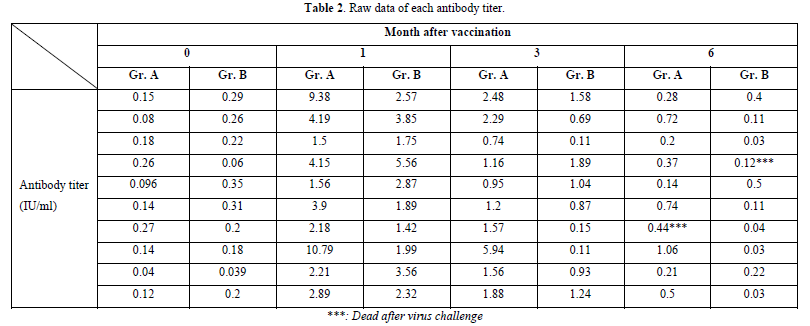
The GMT of RVNA immunity at 0 months, 1 month, 3 months, and 6 months were 0.129 IU/ml, 3.427 IU/ml, 1.652 IU/ml, and 0.387 IU/ml, respectively, with boosters administered at month 1 and month 3 after vaccination. The immunity levels remained and were significantly higher than the pre-vaccination levels at 0 months (P-value = 0.00 and 0.11, respectively). Moreover, the mean immunity at these time points exceeded the cutoff of 0.5 IU/ml. However, at month 6, the mean immunity level decreased below the cutoff, although it remained significantly higher than before vaccination (P-value = 0.01).
In Group A, the survival rate after the challenge virus invaded the muscle was 90% (1 died and 9 survived), with the deceased sample testing positive for Direct Fluorescent Antibody (DFA) (Graph 2).
Group B (Bayovac-R®)
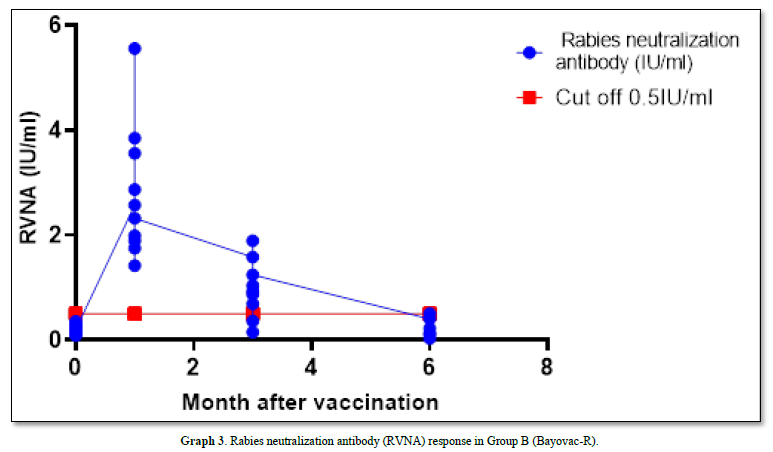
The GMT of RVNA immunity at 0 months, 1st month, 3rd month, and 6 months were 0.204 IU/ml, 2.562 IU/ml, 0.727 IU/ml, and 0.096 IU/ml, respectively. Immunity levels at the 1st and 3rd months remained above the cutoff of 0.5 IU/ml and were significantly higher than a month 0 (p-value = 0.00 and 0.11, respectively). However, at month 6, the mean Nab levels were lower than the 0.5 IU/ml cutoff, and the mean was not significantly different from the levels before vaccination (p-value = 0.333).
In this group, the survival rate after the challenge virus invaded the muscle was 90% (1 died and 9 survived), with the deceased sample testing positive for Direct Fluorescent Antibody (DFA) (Graph 3).
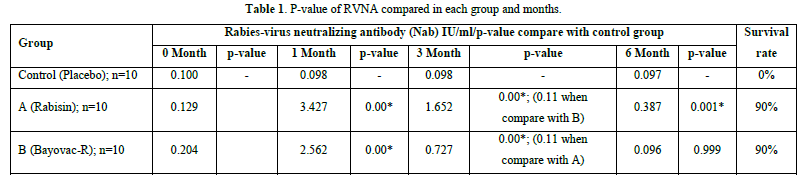
In the comparative analysis of immunity levels between the two vaccinated groups and the placebo-treated control, significant differences were observed. At 1 month after vaccination, both Group A and Group B exhibited a significant increase in Nab levels compared to month 0 (p-value = 0.00). Furthermore, there was no significant difference between Group A and Group B in terms of their ability to stimulate Nab levels (p-value = 0.453). At month 3, both Group A and Group B demonstrated a strong immune response compared to the control group (p-value = 0.00). However, the Nab levels in Group A were significantly higher than those in Group B (p-value = 0.011), indicating that Group B experienced a greater decline in Nab levels. Finally, at the sixth month after vaccination, Group A maintained higher immunity levels than the control group, although the mean value fell below the cut-off (p-value = 0.001). In contrast, Group B exhibited Nab levels lower than the cut-off, and the difference from the control group was not statistically significant (p-value = 0.999) (Tables 1 & 2).
CONCLUSIONS AND DISCUSSION
Based on the results of the three experimental groups, it was observed that both commercial rabies vaccines were capable of eliciting immune responses at protective levels above 0.5 IU/ml at 1 month and during the first 3 months. However, at month 6, both groups exhibited substandard levels of immunity, with a GMT of 0.387 in Group A and 0.096 in Group B. Rabisin demonstrated a higher mean activation and persistence of Nab compared to Bayovac-R at Months 3 and 6. This indicates that booster vaccinations may be necessary after 6 months from the initial vaccination dose to raise the Nab levels above the 0.5 standard, thereby establishing herd immunity and paving the way for a rabies-free community in the future. The recommended protocol in Thailand and other affected areas suggests administering booster injections approximately 1 month after the first dose and continuing annually [14]. This can help maintain Nab levels for 1-3 years. However, in terms of prophylaxis and efficacy, even if the immune level falls below 0.5 IU/ml, animals can survive rabies infection, as evidenced by the experimental results where both groups exhibited a 90% survival rate despite having a mean Nab level below 0.5 IU/ml. In the raw data, several samples survived with Nab levels below 0.5 IU/ml, whereas all unvaccinated hamsters in the control group died (Table 2).
It should be noted that although Rabies-Specific antibodies such as RVNA have the ability to clear the rabies virus from the central nervous system in mice and in vitro cell line experiments [15], this does not guarantee 100% protection. The level of 0.5 IU/ml was established as an indicator of adequate vaccination rather than complete protection. For humans at risk of rabies exposure, the serum neutralization test with a standard challenge virus strain is used to determine a Nab level of 0.5 IU/ml, which is recommended by the WHO. This includes individuals such as laboratory workers, veterinarians, and personnel working with mammals. When the antibody titer drops below 0.5 IU/ml, a booster dose should be administered immediately [16]. Additionally, the Advisory Committee on Immunization Practices (ACIP) recommends complete neutralization of the rabies virus at a serum dilution of 1:5 as the minimum evidence of circulating rabies virus neutralizing antibodies. The interpretation of the titer depends on the specific clinical situation for which the test was requested [17]. This means that an RVNA level of 0.5IU/ml is not a reference to the survival rate. but can be used to refer to the quality of the vaccine A good vaccine must be able to induce BVNA levels up to 0.5 IU/ml as mentioned above.
Animals have different cut-off values of RVNA
Previous studies have demonstrated that each animal has distinct cut-off values for RVNA protection. For instance, in Aubert's challenge, the cut-off values for dogs and cats, which enable them to survive rabies, were determined as 0.2 IU/ml and 0.1 IU/ml, respectively [18]. Similarly, during the 2017 wildlife challenge, where an oral vaccine was tested on wild animals like raccoons, red foxes, and skunks, RFFIT values were measured 28 days prior to the challenge. It was found that the mean RVNA of the surviving animals ranged from 0.25 to 0.5 IU, never exceeding 0.5 IU/ml [19]. Additionally, the CDC also stated, "There is no 'protective' titer against rabies virus. In animal studies, survival against rabies virus infection is often more likely to occur the higher an animal's titer at the time of infection, but it is not a definitive indicator of survival" [5].
Adequate vaccination leads to Rabies-free areas.
Although RVNA levels may not indicate the degree of prophylaxis, the value of 0.5 IU/ml obtained through RFFIT assays remains significant in various sectors. It serves as an indicator of the success of vaccination and the immune response, which ultimately contributes to the establishment of Rabies-free areas [20]. Cooperation among different agencies is essential to control animal movement, conduct continuous immune surveillance, and implement mass vaccination programs for dogs and cats. Similar measures should be taken in humans, such as regular prophylactic vaccination and raising awareness among people using the One Health principle. By implementing these strategies, qualification of Rabies-free areas according to WHO standards can be achieved [21]. Therefore, an RVNA level of 0.5 IU/ml should still be considered. However, it should not be solely relied upon as a measure of 100% prophylaxis. Instead, it should be considered when assessing the degree of protection and when making decisions regarding the distribution and administration of rabies vaccines in different contexts, all with the ultimate goal of preventing rabies itself.
ACKNOWLEDGMENTS
We would like to express our gratitude to the Laboratory Animal Unit of the Faculty of Medicine, KhonKaen University, Thailand, for providing us with Syrian hamsters for use in this project. The authors declare no conflicts of interest.
No Files Found
Share Your Publication :