-
Publish Your Research/Review Articles in our High Quality Journal for just USD $99*+Taxes( *T&C Apply)
Offer Ends On
Banafsheh Shoja, Abbasali Mottalebi, Behrouz Akbari-Adergani* and Noordahr Rokni
Corresponding Author: Behrouz Akbari-Adergani, Water Safety Research Center, Food and Drug Administration, Ministry of Health, and Medical Education, 11136-15911, Tehran, Iran.
Received: May 16, 2023 ; Revised: May 26, 2023 ; Accepted: May 29, 2023 ; Available Online: June 16, 2023
Citation: Shoja B, Mottalebi A, Akbari-Adergani B & Rokni N. (2023) The Effect of Chitosan Coating Enriched with Orange Peel (Citrus Sinensis) Waste Extract on Extending Preservation and Chemical and Functional Properties of Refrigerated Beluga Sturgeon (Husohuso) Fillet. J Vet Marine Res, 3(1): 1-9.
Copyrights: ©2023 Shoja B, Mottalebi A, Akbari-Adergani B & Rokni N. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Views & Citations
Likes & Shares
In this study, chitosan biofilms containing different concentrations of the orange-peel extract (0.5, 1.0, and 1.5%) were prepared and their physical factors including water vapor permeability (WVP), color, and water solubility (WS) were characterized. Enriching the chitosan-based coatings with orange peel extracts improved physical characteristic factors and WVP, color and WS improved for extending preservation of refrigerated beluga sturgeon so that significant difference in these factors were observed between the enriched coatings and control ones (p<0.05). The fish fillets were analyzed for total lipid (3.33 ± 0.41%), crude protein (14.90 ± 1.04%) and total volatile base nitrogen (10.12 ± 1.15 mg N/100 gr) and then subjected to the three treatments as well as blank coatings with 0.0-1.5% levels of orange peel waste extract for 14 days in 4 ⁰C. The results revealed that the biofilm enriched with 1.5% orange peel waste extract was effective on preserving phenolic compounds and maintaining antioxidant properties of fish fillets during preservation period (p<0.05). The findings of this study revealed that chitosan coating enriched with orange peel extract improved physical factors in the coatings and preserved the antioxidant compounds in the fish fillets and maintained their shelf life through two week refrigerated time.
Keywords: Antioxidants, Beluga sturgeon, Chitosan coating, Functional properties, Orange peel extract
INTRODUCTION
Nowadays, fish is an important part of people's diet in developed and developing countries. Fish due to the high digestibility of protein and a suitable combination of essential amino acids like lysine and methionine is essential for good health and nutrition [1]. Health benefits of essential polyunsaturated lipid acids including omega-3 and omega-6 fats stimulate interest in increasing seafood intake. The potent action of autolytic enzymes and microbial activity in fresh fish makes it one of the most perishable foods [2]. The use of fresh fish is in great demand among consumers however, a developing group called green consumers want to use natural foods without chemical additives that are microbial healthy and packaged in appropriate cases in terms of environmentally friendly. Increasing the shelf life of food, reduces the financial and environmental burden of the food industry [3,4]. Therefore, due to food safety and human health and consumers' desire to use natural compounds, the use of healthy compounds with antimicrobial and antioxidant properties can be considered an effective way to control microbial growth and increase the required shelf life. In addition, the potential toxicological effects of chemical preservatives and synthetic antioxidants have led researchers to develop methods to increase the shelf life of perishable foods based on natural compounds with a broad spectrum of antimicrobial and antioxidant activity [5]. Plants, vegetables and even some foods extracts, and essential oils are among the natural compounds that have been studied for their antimicrobial and antioxidant properties [6,7]. Recent studies showed that the use of these compounds in the food industry can be good alternative for synthetic chemical preservatives such as benzoate and sorbate which are common in many foodstuffs [8]. However, their cost of use and other challenges such as odor intensity and potential toxicity for uncontrolled release of these substances into the food products and inactivation of some valuable compounds due to their undesirable reactions to the final products, caused sensible limitation in their use as food preservative [9]. It has been revealed that plant extracts contain active compounds that act specifically on bacterial cells. It seems that their antimicrobial activity is mainly due to hydrophobic nature of the active substances and secondary metabolites.
Combination of natural and inexpensive plant-based waste extracts with special polymers as chitosan can be good choice to reduce the dose of these extracts and preventing their undesirable effects [10]. Previous studies showed that the main advantage of this technology is that it maintains the rate of releasing antimicrobial agents into the product where it is most exposed to bacterial infestation [11]. Chitosan is one of the most important biopolymers ever used to make biofilms and edible coatings [12]. This linear polysaccharide compound is an important renewable resource and pretended as the second most abundant natural biopolymer after cellulose in the world. The ability of renewable films as carriers of antioxidant and antimicrobial compounds and other active agents to control pathogens and improve the quality and shelf life of aquatic animals in the packaging industry is well documented [13]. Citrus fruits and their wastes are of great value as they are rich source of antioxidants and antimicrobials such as glycosidic flavonoids, coumarin, glycosides, beta and gamma-sitosterol, polyphenols and essential oils [14]. These fruits and their by-products are very frequent worldwide because of their affordable economic reach, delicious taste, preferred flavor, and consumers' awareness of the potential health benefits. Orange peel (Citrus sinensis L.) an important source of nutraceuticals such as ascorbic acid, carotenoids, and phenolics is the most widely grown citrus species around the world [15]. The main aim of this study was to evaluate the effect of chitosan coating enriched with orange waste extract on extending preservation and chemical and functional properties of refrigerated beluga sturgeon fish fillets.
MATERIALS AND METHODS
Chemicals and reagents
All the chemicals and reagents were of analytical grade with highest purity. All phenolic compound standard solutions (including ellagic acid, gallic acid, catechol, resorcinol, vanillin, benzoic acid, and ascorbic acid) were prepared from analytical reagent grade chemicals (Sigma-Aldrich, Steinheim, Germany) and ultrapure water which was supplied by purifying water with the UHQ Elga instrument (Millipore, Le montsur-Lausanne, Switzerland). All solvents used in chromatography analyses were HPLC grade and purchased from Merck chemical Co. (Dramstadt, Germany).
Sample collection and preparation
Beluga fish with an average weight of 47-50 kg were purchased from a sturgeon farm in Sari city, Mazandaran province of Iran. The fish was placed on the crushed ice in isolated boxes and transferred to the food chemistry laboratory of the food hygiene department of Islamic Azad University under sterile conditions. Fish were washed with tap water several times and then filleted by hand. The average weight of each fillet was 10.0 g and placed under UV sterilized conditions for 20 min.
Preparation of coating solution
For preparation of the coating solution, 2.0 g of chitosan powder with average molecular weight (Chitosan Poly D-Glucosamine, Sigma-Aldrich, Germany) was added into 100 ml acetic acid solution (1% v/v) and was then stirred by a magnetic stirrer (IKA, Germany, 1200 rpm) at room temperature for 3 h. Glycerol (Merck chemical Co., Dortmund, Germany) was then added to chitosan at 0.75% concentration as a plasticizer and stirred at 60°C for 30 min. Chitosan coating solution was filtrated through a Whatman No. 3 filter paper to remove any un-dissolved particles [16].
Preparation of orange peel extract
Healthy oranges without any fungal contamination were purchased from Sari Citrus Research Center, peeled, and placed in 96% ethanol for 20 min. Then peels were dried in the oven at 60°C for one hour. To increase the contact surface with the solvent, the dried peels were crushed and sieved with a laboratory grinder. In the next step, 50.0 g of the peels was mixed with 300 ml of pure ethanol in a decanter, and after 24 h at room temperature, the alcoholic phase was separated from the plant material with Whatman No. 1 filter paper. Following evaporation of the solvent, the samples were kept at -4°C until further analyses.
Preparation of the biofilm
Orange peel extract was mixed with tween 80 (0.2%) (Sigma-Aldrich, Germany) as an emulsifier and was stirred mildly at 40°C for 30 min so that smooth distribution of emulsifier appeared. Separate treatments of orange peel extract in various concentrations (0.0, 0.5, 1.0, and 1.5%) were added to the chitosan solution and well homogenized by ultra Turrax homogenizer (IKA, Germany) at 20000 rpm for 5 min. The air bubbles were removed by a relative vacuum. The formulated solution was sprayed finely on Nylon polymeric film (Ultrafine spraying, Techno Sanat, Iran). The prepared biofilms were dried at ambient temperature for 72 h and then placed in a desiccator in a dry atmosphere in the presence of magnesium nitrate for 6 h until all the biofilms were dried thoroughly [17].
Treatment of fish fillets
The treatments selected in this study include control test (beluga coated with biofilm-free orange peel extract as blank sample), treatment 1 (beluga coated with biofilm enriched with 0.0% of orange peel extract), treatment 2 (beluga coated with biofilm enriched with 0.5% of orange peel extract), treatment 3 (beluga coated with biofilm enriched with 1.0% of orange peel extract) and treatment 4 (beluga coated with biofilm enriched with 1.5% of orange peel extract). The prepared biofilms were categorized into five distinct groups and were stored in refrigerator at 4 ± 1°C. The physicochemical and antioxidant properties of samples were analyzed on the 1st, 5th, and 14th days during shelf life. For each treatment, five different batches were considered randomly to achieve better statistical data and analysis [18].
Chemical Analyses
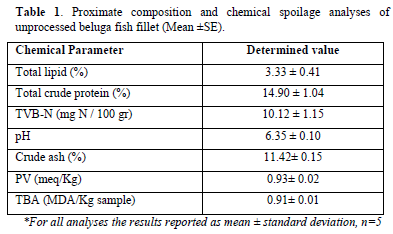
Proximate composition and some chemical spoilage indices such as protein, lipid, ash, peroxide value (PV), thiobarbituric acid (TBA), and total volatile base nitrogen (TVB-N) were determined in unprocessed beluga fish. The total crude protein was determined by Kjeldahl’s method with a multiplier 6.25 coefficient [19], Soxhlet method was used to determine the amount of lipid [20] and the crude ash was determined through mineralization of the samples at 550°C [21]. Lee method was used for determining the amount of PV [22]. The TBA was determined according to the AOAC method [21]. Vapor distillation was used for the determination of TVB-N [21]. The measurement of pH for each case was carried out using a digital AZ-Metrohm, 780 pH Meter (Germany) equipped with a combined glass-calomel electrode on 10 g of homogenized sample in distilled water (1/10 v/v, sample/water) [23].
Biofilm Water Vapor permeability
Biofilm water vapor permeability (WVP) was determined using specific glassy vials. At first, 8.0 ml of distilled water was added to create 100% humidity in the vial atmosphere. The biofilm samples were placed on entrances of vials and sealed by rubber O-ring and clamped tightly. The initial weight of all biofilms was recorded by an analytical balance (Sartorius, Germany) and then placed in a desiccator containing silica gel to produce a relative humidity of near zero using anhydrous calcium sulfates. The samples were weighed every hour for up to 7 h. Finally, the weight loss and then water vapor permeability rate (WVPR) was calculated using the following formula:
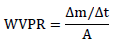
Where ∆m is the weight loss (gr), ∆t is the difference between initial and final time (s) and A is the film surface (m2) [24]. Then the WVP was calculated and reported as gr/m.s.pa based on the recent calculations:
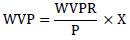
Where X is the film thickness (m), and P is the vapor pressure for pure water at 25°C which was assumed as 3169 pa.
Water solubility of biofilms
One square centimeter (cm2) of biofilm pieces was exactly separated and weighed before placing them in a dry desiccator overnight. These biofilm pieces were placed in 100 ml of deionized water and agitated on a magnetic stirrer (IKA, Germany) for 24 h. Then, the biofilms were separated from the aqueous phase and placed at 40°C up to reach constant weight. The solubility value for biofilms was calculated using the following equation:
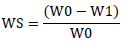
Where W0 is the initial weight of biofilm, W1 is the final weight of dry biofilm and WS is the water solubility value [25].
Biofilm colorimetry
The colorimetry test on the biofilms was performed through diffuse reflection spectroscopy (DRS) by a solid UV spectrometer (Shimadzu, Japan). All the spectrums were recorded from 190 to 800 nm and the effect of enriching with orange peel waste extract on the profile of ultraviolet and visible spectrum was evaluated [26].
Detection of phenolic compounds by HPLC
A Dionex HPLC apparatus was used for the recording profile of the phenolic compounds in preserved fish fillet samples. This chromatographic system was composed of a high-pressure pump and a UVD 170U multi-wavelength detector which was adjusted at 254 nm. A Rehodyne7725i injector with a 25 µl loop was used to inject the phenolic fish-extracted sample solutions into the analytical column (ODS, 4.6*250 mm, 5 µm). For the mobile phase, a degassed mixture of methanol and acetic acid (75% in water) was prepared and delivered at a flow rate of 1.0 ml/min. The elution of the column was performed with 100% methanol for 5.0 min and the acetic acid phase was increased up to 50% for 7.0 min and continued up on finishing the elution program. HPLC data were acquired and the area under the curve (AUC) for each phenolic compound was processed using Chromeleon ver. 6.60 software. These data were assumed as a signal which is proportional to the concentration of each phenolic compound [27].
Statistical analyses
Data analysis was done in SPSS software version No. 18 (SPSS Inc., Chicago, IL, USA). All the experiments were performed in triplicate, preparing coating treatments was performed in five different batches and a completely randomized design was used for statistical analyses. The statistical analysis was done by using a one-way analysis of variance (ANOVA) followed by Duncan’s multiple-range tests. A p-value of less than 0.05 was considered significant.
RESULTS AND DISCUSSION
Proximate compositions and chemical spoilage indices
Chemical quality of collected fish samples and their suitability for storage and final consumption was evaluated for proximate compositions and some chemical spoilage indices. In Table 1 the results for the examined factors were demonstrated. Beluga sturgeon fish due to its nutrient composition is an important marine food source candidate for human nutrition. The TVB-N mean value in the examined samples was 10.12 mg N/100 g while 25-35 mg N/100 g is usually regarded as the upper limit of acceptability for human consumption [28]. The initial proximate analyses showed that the fish samples can be assumed as an appropriate nutrient medium of lipid and protein for consumers. These results are compatible with Bongiorno [29] which regarded sturgeon as a source of protein, with high lipid content. Furthermore, the mean pH values of the samples were determined as 6.35 in the raw sample which was according to the standard pH value that should be between 6.00-6.50 for fresh fish. The upper limit of acceptability was considered as 6.80-7.00 [30,31]. In addition, the peroxide value (PV) and 2-thiobarbituric acid (TBA) values were 0.93± 0.02 meq/Kg and 0.91± 0.01MDA/Kg, respectively, that were by far lower than recommended limit values [32]. Recently, Shahhoseini [33] evaluated the antioxidant effects of Pullulan edible coating with Nasturtiumn officinale extract on the chemical corruption of fresh beluga sturgeon fillet during storage in a refrigerator temperature and showed that Pullulan coating as well as1000 ppm watercress extract significantly postponed lipid oxidation by decreasing PV and TBA production in the sample matrix and it had the lowest total volatile basic nitrogen and pH (p<0.05) [33]. These findings are in line with the findings in this study and obviously indicating the effectiveness of the coating material on shelf life of beluga sturgeon fillets.
Water vapor permeability
The effects of the film-forming solution enriched with different levels of orange peel extract were shown in Table 2. The obtained results showed that the biofilm coatings with more level of orange peel extract have lower WVP than the control treatment, significantly (p<0.05). The lower WVP for biofilms with more orange peel extract concentrations seems to be consequence of the high viscosity of the film-forming solutions [34]. Spraying the orange peel extract with more concentration on the nylon polymeric substrate, resulting in the formation of more strong gels. This itself made difficult the removal of all air bubbles in the permeability test and hence lead to the lower WVP and improving preservation of embedded product. Similar results had been reported in previous studies showing that polymeric solutions with significantly higher viscosity had lower WVP [35,36]. According to Table 2 results, any differences in orange peel extract concentration generated obvious changes in permeability properties for the resulting biofilm coatings.
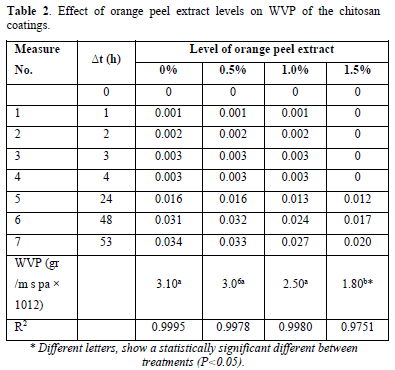
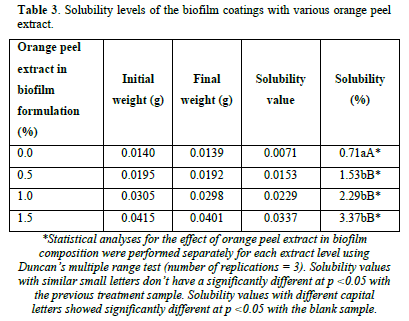
Solubility characteristics
Solubility in water, especially for fish packaging, is an important property of food-coating biofilms. Industrial applications may require water insolubility to enhance product integrity and water resistance. However, in some cases improving film water solubility with natural compounds before final consumption of the product might be beneficial [37]. Evaluating the quality of treated nylon biofilms revealed that film pieces maintained their integrity after reaching constant weight at 40°C. The results showed that there was no significant difference between the treatment coatings with each other, while the solubility percent showed a significant difference between the treatments and control coating (p<0.05). In natural orange peel extract, there are various water-soluble compounds and so increasing its concentration in biofilm formulation can increase its solubility [38]. The lower values of solubility for control treatment with no orange extract in the biofilm formulation should be attributed to chitosan and its water-insolubility properties (Table 3). In a similar study, Gao [39] reported that the presence of water-soluble tea polyphenols could increase the water solubility of chitosan and corn starch-based films and the higher hydrophilicity of tea polyphenols may be responsible for the greater interaction between the film matrix and water [39]. In their study, the gradual increase in the concentration of tea polyphenols resulted in increasing the water solubility of the blended film which is consistent with findings in current study. Water-soluble derivatives of chitosan have also been reported to be effective in food, paints, and water treatment applications [40,41].
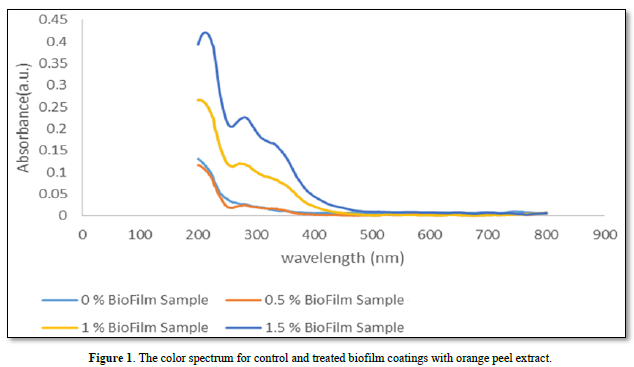
Biofilm colorimetry
The results of the colorimetry test through diffuse reflection spectroscopy revealed that an obvious increase in absorption band at 280 nm. Increasing the concentration of the orange peel extract from zero up to 1.5% in biofilm coatings, caused a dominant increase in absorption intensity from zero to 0.225 nm in the recorded spectrum (Figure 1). This colorimetry test was repeated three times and the standard deviation for absorption of color was less than 3.0 percent for each biofilm. This finding confirmed the homogeneity of the orange peel extract on the surface of the biofilms in all cases which were enriched by orange peel extracts. Lemes [42] reported that there are some interactions between the chitosan and the compounds of the methanolic fraction of Euphorbia umbellate extract [42]. The increase in the concentration of the extract added to the membranes resulted in a sharp change in the coloration of the membranes to a darker brown color. Some other studies revealed that chitosan membranes incorporated with extracts containing phenolic compounds had similar behavior [43,44]. This may be due to the interaction of the amine groups in the chitosan and carboxylic groups in the extract compounds. It has been shown that phenolic compounds (such as gallic acid) contain carboxylic groups and could interact with chitosan, similarly [44].
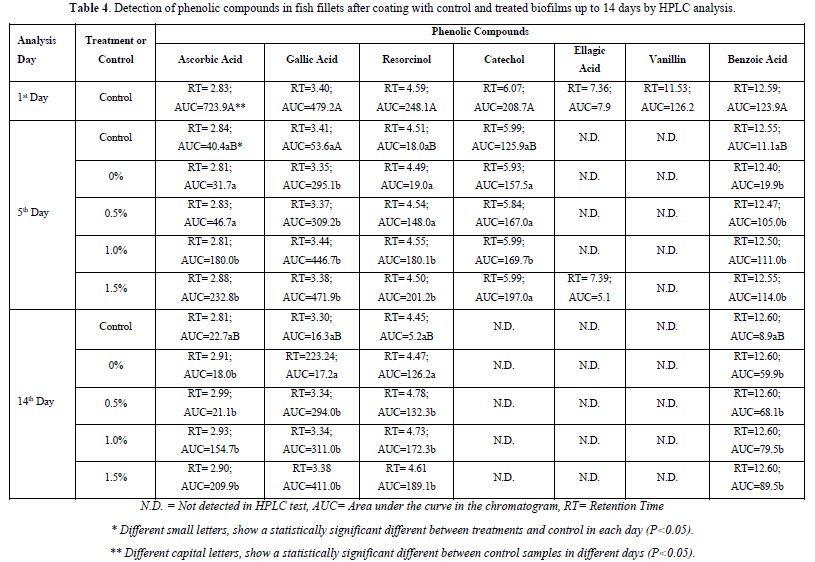
Preserving phenolic compounds and antioxidant property
Results for control of phenolic compounds level in refrigerated beluga sturgeon samples during storage period which were embedded in various treatments and control biofilm coatings illustrated in Table 4. Seven phenolic compounds including ellagic acid, gallic acid, catechol, resorcinol, vanillin, benzoic acid, and ascorbic acid were detected in beluga fish fillets via HPLC analysis and recorded their variation level through integrating area under the curve (AUC) for each phenolic compound peak in the chromatogram. As can be seen in the presented data in Table 4, the HPLC signals for all phenolic compounds had a slight decrease after initial storage in biofilm coatings, except for vanillin which was not detected in the experiments after first control in 5th day and ellagic acid which was only detected in the 1.5% treatment biofilm coating in the 5th day and not detected in other treatment and control biofilm coatings in this evaluation. As can be seen in Table 4, there was a significant difference between the recorded AUC signals in treatments and control biofilm coatings in each day for ascorbic acid, gallic acid, resorcinol, catechol, and benzoic acid through storage time (p<0.05). Also, the data in this table clearly shows the decrease in the HPLC signal of the control sample on different days which were taken under experiment. The obtained results clearly show the inappropriateness of the coating without chitosan and orange-peel extract for preservation of main detected phenolic compounds (except for gallic acid after 5 days from first coating by the biofilm) and maintaining antioxidant property of fish fillets during the period of 14 days even in the condition of storage in refrigerator temperature. The obtained results also showed that the effect of orange peel extract in the biofilm coating, particularly in 1.5% level on the preservation of phenolic compounds was significantly attributed to gallic acid, ascorbic acid, and benzoic acid with maintaining higher level of these phenolic compounds. The obtained data was also showed that AUC in each chromatogram had a direct proportional increase with percentage of orange peel extract up to 1.5% which indicates the improvement in preserving of phenolic compounds in the biofilm coatings with high levels of orange peel extract. Evaluating obtained results after five days from initial preservation showed that any increment in the amount of orange peel extract from zero to 0.5%, 1.0% and 1.5% were effective in preserving ascorbic acid, gallic acid, resorcinol, catechol and benzoic acid by 5.7, 8.8, 11.2, 1.5 and 10.3 times, respectively. The similar results were recorded for after analyses of data in 14th day which effectiveness of increment in orange peel extract were 9.2, 25.2, 36.7, N.D and 10.0 times, respectively for the mentioned phenolic compounds in comparison with the control coating film. These findings are in line with recent previous report in which antioxidant properties of herbal based coatings were attributed to their phenolic compounds [45]. In general, the biofilm coating enriched with 1.5% orange peel extract after five days from initial storage of beluga sturgeon fillet at refrigerator temperature was 32%, 98%, 81%, 94% and 92% effective in preserving the major phenolic compounds which were identified in the fillet (ascorbic acid, gallic acid, resorcinol, catechol and benzoic acid, respectively). These results were 29%, 85%, 76%, N.D. and 72% for preserving major phenolic compounds and antioxidant property in Husohuso fillet in comparison with control coating after 14 days of initial storage at 4°C.
CONCLUSIONS
On this study, chitosan-based biofilm coatings enriched with orange peel extract was prepared with improved beneficial water vapor permeability, color, and maintaining water solubility. The enriched biofilm had the preservative properties for antioxidants and has excellent role in increasing the shelf life of refrigerated sturgeon fish fillets. The formation of chitosan-based biofilm coatings enriched with orange peel extract was imparted with beneficial water vapor permeability, color, and maintaining water solubility. This study also showed that the enriched biofilm had the preservative properties for antioxidants and can impart desired flexibility and did not significantly affect the permeability of WVP biofilms. Based on the obtained results, the effect of orange peel extract in all coating treatments was significant on preserving phenolic compounds (p<0.05) and the treatment enriched with 1.5% orange peel extract showed the best performance in preservation of phenolic compounds at 4°C.The findings of the study also showed that chitosan biofilm coating containing orange peel extract has excellent role in increasing the shelf life of refrigerated sturgeon fish fillets and preserving phenolic compounds. Due to the proven antioxidant and antibacterial activity, this extract can be used as an appropriate alternative to chemical preservatives to increase refrigerated fish fillet shelf life. It can be concluded that orange peel extract as a natural food additive that has the potential to serve as a useful alternative to synthetic antioxidants can be used for improving the safety and quality of fish and fish products.
No Files Found
Share Your Publication :