-
Publish Your Research/Review Articles in our High Quality Journal for just USD $99*+Taxes( *T&C Apply)
Offer Ends On
Jason Kwok and Mike Sabeti*
Corresponding Author: Mike Sabeti, Advanced Specialty Program in Endodontics UCSF School of Dentistry, Parnassus Ave San Francisco, USA, Tel: 2812355967; E-mail: Mike.sabeti@ucsf.edu
Received: July 31, 2021 ; Revised: September 21, 2021 ; Accepted: October 14, 2021 ; Available Online: December 28, 2021
Citation: Kwok J & Sabeti M. (2021) Return of Sensibility After 7 Years in a Traumatized Tooth Treated by The Regenerative Process: A Case Report. J Oral Health Dent Res, 1(1): 1-6.
Copyrights: ©2021 Kwok J & Sabeti M. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Views & Citations
Likes & Shares
Background: In this case report we describe a case of successful regenerative treatment in which sensibility was recovered seven years after the regenerative process.
Case description: An immature maxillary right central incisor (tooth #11) with a necrotic pulp and a periodical radiolucency in a seven-year-old patient was treated using a regenerative protocol. Bleeding was inducted into the canal system and mineral trioxide aggregate was place over the blood clot followed by a permanent composite restoration. Radiographic examination showed resolution of the periapical pathosis, continued development of the most apical portion of the root and finally closure of the root apex.
Practical Implication: This case exemplified that re-establishment of a sensory component of the tissue formed in the root canal after a regenerative procedure is possible years after the treatment is completed. In addition, the regenerative procedure does produce continued development of root dentin and prevention or healing of endodontically related apical periodontitis.
Keywords: Regeneration, Revascularization, Trauma
INTRODUCTION
Treatment of the immature permanent dentition after traumatic incidents represents a unique challenge in endodontics. Open apices and the potential for fracture of thin dentinal root walls are just a few of the challenges faced by clinicians managing immature teeth with necrotic pulps. Traditionally, calcium hydroxide (Ca(OH)2) was used to induce hard tissue formation and an apical barrier. Apexification using Ca(OH)2 does result in a barrier across the open apex but not with the continued development of root dentin. In addition, a sequela of apexification is the higher likelihood of fracture of the remaining thin root dentin walls [1].
After the development of mineral trioxide aggregate (MTA) in the early 1990s, the use of this bio ceramic material supplanted the use of Ca(OH)2 as a material of choice in open apex teeth. It reduced appointment times and produced true closure of open apices [2]. The barrier technique, however, did not allow continued development of an immature tooth. Hence, the tooth was subject to a higher likelihood of fracture [3].
The continued development of an immature tooth has long been thought to be beneficial for the longevity and prognosis of a tooth [4]. The concept of pulpal regenerative was first introduced in the 1960s by Nygaard-Ostby [5,6]. Through histological evaluation, he discovered hard tissue deposition on the thin root walls of the canal. The hard tissue deposition occurred by induction and migration of stem cells into the canal space. With proper stimulation, modifying growth factors and appropriate scaffolding, the stem cells differentiated and formed a pulp-dentin-like complex and revascularized the tooth [7-10].
Successful regenerative results in prevention or healing of apical periodontitis and the continued development of strong root dentin [11-13]. There are numerous case reports on regeneration, but reports with a return of sensibility are limited [11,14-19]. Moreover, there are very limited reports to date [20] that have demonstrated sensory function returning to the tooth many years after the regenerative process has been completed.
The purpose of this case report is to highlight a highly unusual case in which sensory function was recovered years after the regenerative process was completed in an immature tooth which had a necrotic pulp and long-term development of the most apical portion of the root occurred.
CASE REPORT
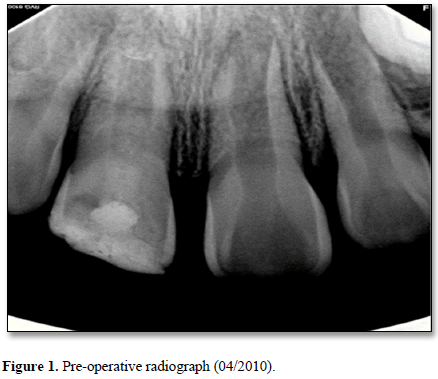
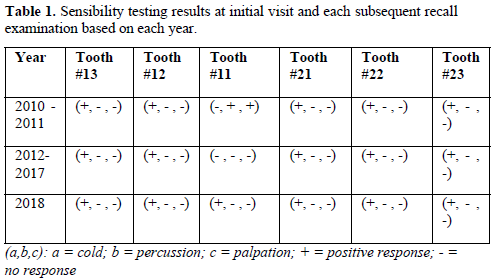
A seven-year-old Filipino female presented with her grandmother to the Dental Clinic at the University of California San Francisco School of Dentistry in April 2010 with intermittent pain associated with the right maxillary central incisor (tooth #11). The tooth had a history of a complicated crown fracture. The gingival around the tooth was inflamed. Sensibility testing at the initial visit resulted in a no response to cold and positive responses to both percussion and palpation. Electronic pulp testing resulted in a negative response. Radiographic examination showed an open apex, periapical radiolucency, a complicated crown fracture and an interim restorative material in the coronal portion of the pulp space (Figure 1). An initial diagnosis of necrotic pulp with symptomatic apical periodontitis was made. Local anesthesia with 1.8mL of 2% lidocaine HCl with .17mg of epinephrine was administered. Rubber dam isolation was achieved and access was completed. Pulpal debridement using only hand files was performed using 20 mL of 5.25% sodium hypochlorite (NaOCl) as an irrigant for 5 minutes and Ca(OH)2 (Ultracal® Dentsply Tulsa Dental, Tulsa, OK) paste as an intermediate medicament followed by a glass ionomer temporary material. Two weeks after the initial appointment, pulpal regeneration was recommended and accepted by the patient’s grandmother.
Unfortunately, the patient failed to appear for her next two appointments. Finally, in January 2011, the patient began the regenerative treatment on the tooth (tooth #11). At this appointment, the patient presented with a draining sinus tract. Clinical examination and sensibility testing with cold as well as the electronic pulp testing resulted in a new diagnosis of necrosis of the pulp with chronic apical abscess. Local anesthesia with 1.8mL of 2% lidocaine HCl with .017 mg epinephrine was administered. After rubber dam isolation, access was completed and a size 10 K-file was taken to a working length determined with an electronic apex locator. A periapical radiograph was taken to confirm the working length. A K-file was used to lightly abrade the canal walls to disrupt any bacterial biofilm. 20 mL of Sodium hypochlorite 5.25% was used as an irrigant in conjunction with 20 mL of 17% ethylenediaminetetraacetic acid (EDTA) for 5 minutes. The canal was dried using sterile paper points. A triple antibiotic paste containing a mixture of 500 mg of metronidazole, 200 mg of ciprofloxacin and 100 mg of minocycline was placed in the canal up to the level of the cement enamel junction (CEJ) followed by a glass ionomer temporary material in accordance with the new treatment protocol recommended by Banchs and Trope [21].
The patient returned in three weeks for a follow-up clinical exam to ensure normal post-treatment progress. The patient was anesthetized with 1.8 mL of 3% mepivacaine HCl without epinephrine, the tooth isolated with a rubber dam and the field disinfected with 2.5% NaOCl. After accessing the canal, the medicaments were removed by irrigating with sterile saline (20 ml for 1 minute) followed by a flush of 17% EDTA (20ml for 5 minutes) and a final flush with sterile saline (20 ml for 1 minute). Bleeding into the canal up to a level 4 mm below the cement enamel junction was achieved by a pre-bent size 25 K-file introduced several millimeters into the periapical tissues through the open apex. A premeasured piece of Collaplug (Zimmer Dental Inc., Campbell, CA) was carefully placed in contact with the blood clot followed by 2-3 mm of MTA (Grey ProRoot® MTA, Dentsply Tulsa Dental, Tulsa, OK) and a glass ionomer filling material.
Five days later the patient presented to the dental clinic with a persistent sinus tract and draining exudate. After anesthetizing the patient and applying a rubber dam, the MTA was removed and the canal system irrigated with 20 mL of 5.25% NaOCl for 5 minutes followed by 20 mL of sterile saline. Then, Ca(OH)2 paste was placed into the canal as an intracanal medicament up to the level of the cement enamel junction.
The patient returned after one month for clinical examination and assessment. The patient was asymptomatic and the sinus tract had closed. At this appointment, the regenerative procedures were completed once more following the same protocol as the first attempt. The tooth was restored with MTA, followed by glass ionomer and finally a composite restoration.
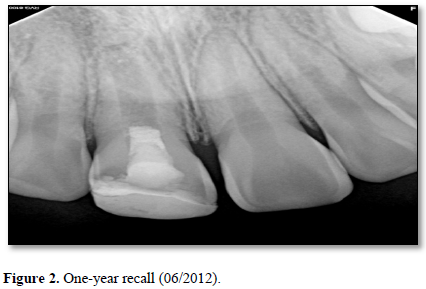
At the six-month recall, sensibility testing with cold was negative and electric pulp testing resulted in a negative response. Periapical radiographs revealed a developing root apex with increased root dentin length and signs of apical closure. At the one-year recall, all sensibility tests were negative again. The periapical lesion had healed and the developing root apex was again apparent (Figure 2).
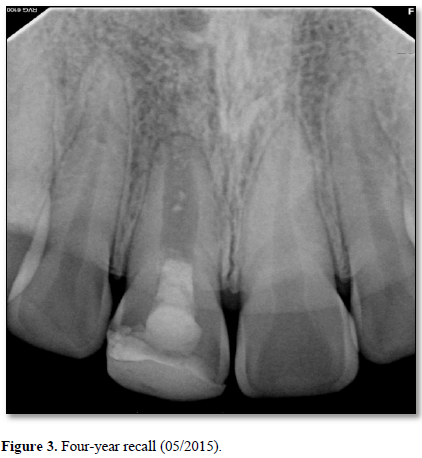
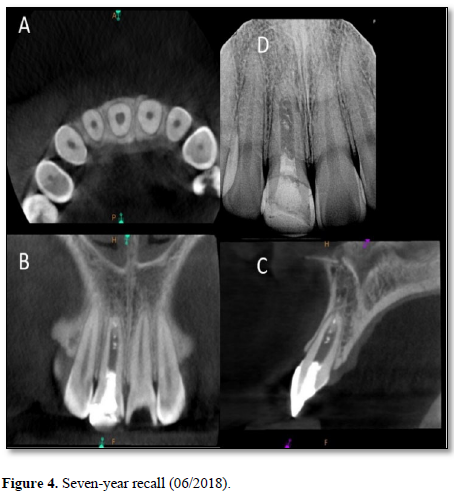
At the three-year recall, sensibility tests with cold were negative, electric pulp testing resulted in a negative response and periapical radiographs showed complete closure of the apex and continued root development in the most apical portion of the root with an intact periodontal ligament (Figure 3). At the four-, five-, and six-year post-treatment examinations, sensibility tests were negative and radiographs showed normal apical tissues with continued apical root development and an intact periodontal ligament. At the seven-year recall, periapical radiographs revealed normal apical tissues with an intact periodontal ligament (Figure 4). Sensibility testing was completed a positive response to the electric pulp test and a strong response to the cold stimulus was noted. Percussion and palpation testing was unremarkable (Table 1). However, at this appointment, the patient presented with a loss of a portion of the coronal restoration and the presence of recurrent decay in tooth #11. After the removal of the decay, the MTA was found to be dislodged and clinical examination revealed the appearance of a reddish tissue inside the coronal portion of the pulp.
Subsequently, MTA was then placed followed by glass ionomer and composite restoration material.
DISCUSSION
The then new treatment protocol proposed by Banchs and Trope [21] focused on several key components used to aid neuro-vasculogenesis and maturation of the root: 1) the elimination of bacteria, 2) the stimulation of growth factor, 3) the release of signaling molecules and 4) the migration of dental pulp stem cells into the root canal system. The presence of bacteria in an immature root canal system will inhibit the regenerative process by initiating an inflammatory osteoclastic process, which can lead to tissue necrosis if bacterial proliferation occurs, delaying root development of the tooth [22].
It is widely accepted that root development in an immature tooth is mediated by Hertwig’s epithelial root sheath cells [23]. Therefore, in order to have continued development of the root, it is imperative that the treatment procedures do not disrupt the ability of these cells to function properly. Consequently, in order to properly disinfect, preserve the integrity of the apical cells, and avoid caustic injury in an immature tooth, a lower concentration of NaOCl [24] in conjunction with a triple or double antibiotic paste as an intermediary medicament is currently recommended [25-28]. In addition, the use of ethylenediaminetetraacetic acid (EDTA) has been shown to release numerous growth factors and signaling molecules that facilitate the proliferation of stem cells [29].
The presence of growth factors and signaling factors help lay the foundation for proliferation of stem cells [30]. The importance of pulpal regeneration is driven by stem cells. The source of these stem cells may be of pulpal origin (DPSCs), exfoliated deciduous teeth (SHEDs), apical papilla (SCAP), periodontal ligament (PDLSCs), and tooth germ progenitor cells (TGPCs) [30]. These stem cells are known to differentiate into a wide variety of cell types, including osteogenic/odontogenic, adipogenic, chondrogenic, myogenic, and neurogenic cells [31-33]. The pathway the DPSCs differentiate into is controlled by the growth factors and signaling molecules [29,34]. Therefore, ideal regenerative outcomes should involve an eclectic mix of all the cells that comprise the dentin-pulp complex, including neurovascular components.
There is a paucity of reports that present both successful regenerative with continued root development and the return of sensibility, especially after an extended period of time. The unique feature in this case report is the return of sensibility seven years after the initial treatment. In order to have sensibility in a tooth, functioning sensory neurons need to be present to send impulses to the brain. In regenerative, coronally migrated DPSCs are capable of developing into sensory nerve fibers, as demonstrated by Nakashima [34] and Arthur [35]. validated the differentiation potential of adult human dental pulp stem cells into functionally active neurons in as little as seven days. Therefore, the capability of sending out nerve impulses can occur in as little as one week after differentiation. The timeline for this process is dependent on whether there have been a sufficient number of stem cells recruited with neurogenic potential and the presence of appropriate signaling molecules to facilitate neurogenesis [36]. For our patient, however, this phenomenon happened to take seven years to occur. An explanation for this exceedingly long time for the sensory function to be restored to the tooth is enigmatic. However, considering the importance of stem cells and the presence of appropriate growth factors and signaling molecules in neurogenesis, it can be concluded that their role was crucial in this case, regardless of the uniquely long timeline.
The overall success in this case can be attributed to the strong correlation between the maturation stage of the tooth and the amount of viable apical pluripotent stem cells. When the patient first presented to the clinic, she was seven years old. Her central incisor at that time was very immature with a wide-open apex. Therefore, the number of viable stem cells present near the apical region of her tooth were likely much greater as compared to a mature tooth with a closed apex [37]. Regeneration has the advantage over traditional apexification since it results in the prevention or healing of apical periodontitis, continued thickening of root dentin, apical closure and, as in this case, the possibility of neurogenesis leading to the restoration of sensory function [38, 39]. On the negative side, dentinal thickening of the root canal wall did not occur coronal to the area of apical closure. Although wall thickening did not occur, the formation of islands of calcification in the canal space is strongly suggestive that vital tissue capable of forming hard tissue occupied that space (Figures 3 & 4). Further research is required to better understand why this was the case.
It is acknowledged that the young age of the patient in the earlier years of the recall examinations may have possibly influenced the results of sensibility testing. At a young age, patients can present imagined or expected responses rather than actual ones due to fear, introversion or a rich imagination. This can lead to inaccurate or erroneous responses. Therefore, because sensibility is a subjective observation, the patient may not have accurately reported a true experience.
For this case, a successful outcome was defined as an asymptomatic tooth with complete healing of a periapical pathosis and apical root closure. The highly unusual finding of sensibility return after seven years was a post facto addition to the definition of success.
In conclusion, this case exemplified the multiple advantages that regenerative has over traditional apexification. It was apparent that the new treatment protocol recommended by Banchs and Trope did promote continued maturation of the root, albeit in altered form, and allowed the process of neurogenesis and restoration of sensory function to occur.
REFERENCES
No Files Found
Share Your Publication :