-
Publish Your Research/Review Articles in our High Quality Journal for just USD $99*+Taxes( *T&C Apply)
Offer Ends On
Nandita Gautam*, Syed Owais Ahmed, Divyanshu Thapliyal and Sohini Dingal
Corresponding Author: Nandita Gautam, Department of Public Health Dentistry, Varun Arjun Medical College and Rohilkhand Hospital, Banthara, Shahjahanpur, India.
Received: June 06, 2023 ; Revised: June 08, 2023 ; Accepted: June 11, 2023 ; Available Online: June 22, 2023
Citation: Gautam N, Ahmed SO, Thapliyal D & Dingal S. (2023) Clinical Validation of the Efficacy of a Mouthwash Comprising Propolis for the Prevention and Treatment of Plaque and Gingivitis: A Phase II Trial. J Oral Health Dent Res, 3(3) 1-6.
Copyrights: ©2023 Gautam N, Ahmed SO, Thapliyal D & Dingal S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Views & Citations
Likes & Shares
The purpose of the present research was to demonstrate the clinical efficacy of an alcohol-free mouthwash comprising 5.0% (W/V) Brazilian green propolis (MGP 5%) for plaque and gingivitis control. A clinical trial phase II investigation included 25 people, men and women aged 18 to 60 years old (35 9), who had a minimum of 20 sound natural teeth, a mean plaque index of at least 1.5 (PI), and a mean gingival index of at least 1.0 (GI). Participants were told to rinse with 10 mL of mouthwash test for 1-minute following brushing their teeth in the morning and at night. When utilizing mouthwash for 45 and 90 days, there was a considerable reduction in plaque and gingival index. These decreases were 24% and 40%, respectively (P.5). There were no significant negative effects in the mouth's soft or hard tissues. The MGP 5% demonstrated efficacy in lowering PI and GI in this trial. To validate such efficacy, however, a double-blind, randomized clinical investigation is required.
Keywords: Alcohol free mouthwash, Brazilian green propolis, Double-Blind, Gram-Positive and Gram-Negative bacteria
INTRODUCTION
The use of mouth rinse as a formal practice is attributed to Chinese medicine around 2700 B.C.E. to heal infections in the gums [1,2]. Gram-positive and Gram-negative bacteria in oral biofilms generate a variety of compounds that cause gingival inflammation (i.e., gingivitis). Periodontitis, a disorder in which gingival and bone tissues are damaged, can be caused by gingivitis. A significant number of people may not conduct mechanical eliminating plaque adequately. Thus, antimicrobial mouth rinses that improve regular home care may be an efficient way of eliminating or managing bacterial plaque and thereby limiting gingivitis and periodontitis [3].
The primary purposes pertain to the enhancement of dental health (particularly plaque and gingivitis reduction) or avoiding the development of infections triggered by oral cavity microbes in particular circumstances such as tooth extraction, intraoral surgical operations, or immune suppression due to cancer therapy or transplantation. Antimicrobial mouth rinses have been recommended to lower oral bacteria stages, particularly Streptococcus mutants [4]. Indeed, chemotherapeutic mouth rinses have been found to be a beneficial complement to routine brushing and flossing for individuals with gingivitis, offering a clinically significant benefit in plaque and gingivitis decrease [3]. Propolis is an adhesive material that honeybees gather from the buds and discharges of particular trees and plants and store in their hives. It has been used in traditional medicine to cure a variety of diseases since ancient times [5]. Propolis' antimicrobial activity is an essential feature, and people have utilized it for ages for its therapeutic characteristics [6]. Propolis has antimicrobial activity due to flavonoids, aromatic acids, and esters found in resins. The most potent flavonoids agents versus bacteria are galangin, pinocembrin, and pinostrobin. Ferulic acid and caffeic acid also aid in propolis's antibacterial activity [7].
The American Dental Association (ADA) Guideline (Council on Scientific Affairs) are applied to products that use chemotherapy drugs to reduce gingivitis and, if relevant, supragingival dental plaque. These regulations do not apply to products that treat gingivitis simply through the mechanical elimination of plaque. Since plaque is the primary cause of gingivitis and other oral diseases, this council suspects that the only embraced chemotherapeutic items that may provide plaque control or plaque alteration asserts will be the ones that additionally show a significant effect against gingivitis. If the item can only exhibit a significant plaque reduction without also demonstrating a substantial decrease in gingivitis, it will be rejected [8].
Consequently, the goal of the present investigation was to demonstrate clinical proof in participants of an alcohol-free mouthwash comprising Brazilian green propolis for plaque and gingivitis control during a three-month period.
METHODOLOGY
Design Study and Product Tested
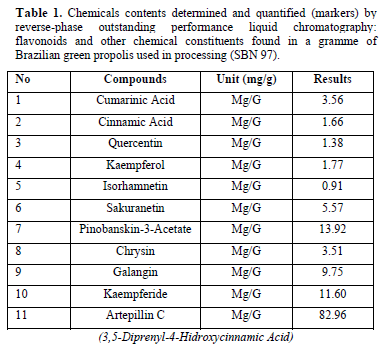
The following was a three-month follow-up phase II interventional investigation carried out at the Department of Public Health Dentistry, Varun Arjun Medical College and Rohilkhand Hospital, Banthara, Shahjahanpur from August 2022 to April 2023. The alcohol-free mouthwash containing 5% w/v Brazilian green propolis (MGP 5%) used in that study was handled by Pharma Ne'ctar in accordance with our request, in accordance with ANVISA [9] and in accordance with ISO 9001 and GMP International. A solution including glycerin, sodium benzoate, and filtered water was supplemented with 5% propolis dry extract (w/v). The quantity in mg/g of the principal chemical markers discovered in green propolis employed in the recipe of the mouthwash research is shown in Table 1.
Participants
The investigation comprised 25 people ranging in age from 18 to 60 years (median age 35 9), in generally excellent health, not pregnant or breastfeeding, and who matched the criteria for inclusion as follows: a minimum of 20 healthy, natural teeth; a mean plaque index (PI) of at least 1.5; and a mean gingival index (GI) of at least 1.0. Participants having orthodontic appliances or removable prosthesis, soft or hard oral tissue tumors, and extensive periodontal disease, as well as those taking antibiotic therapy two weeks prior to the start of the trial, were disqualified. Third molars and teeth with cervical restorations or prosthetic crowns were not counted as teeth. The subjects were chosen based on their availability to participate in the study while it was being done. When the investigation began, all subjects read and signed informed consent forms. The local ethical review committee-the Committee of Bioethics in Research at the Department of Periodontology, Institute of Dental Sciences, Bareilly-approved the investigation's protocol.
Assessing Mouthwash
The preliminary evaluation entailed a full soft and hard tissues inspection to register the current state of the oral mucosa, so that any modifications throughout the period of the research could be noted, and if these modifications could be attributed to the mouthwash. Gingivitis of the mesiobuccal, midbuccal, distobuccal, mesiolingual, midlingual, and distolingual of all eligible teeth was assessed using the Talbott modification Gingival Index of the Lo e-Silness [11], with the gum scored on a four-point scale ranging from 0 (no inflammation) to 3 (severe inflammation). The Turesky adaptation of the Quigley-Hein Plaque Index [10] was used to score the supragingival plaque of the mesiobuccal, midbuccal, distobuccal, mesiolingual, midlingual, and distolingual of all eligible teeth. Plaque used to be graded using an erythrosine 3% solution on a six-point scale ranging from 0 (no plaque) to 5 (plaque covering two-thirds or more of the tooth surface). Every tooth was separated into six sections, three buccal (mesiobuccal, midbuccal, and distobuccal) and three lingual (mesiolingual, midlingual, and distolingual), and plaque was measured using the Turesky adaptation of the Quigley-Hein Plaque Index [10]. Severity Plaque Index and Severity Gingival Index were also examined [12,13]. These indexes calculated the rate of an area that had a high count of plaque (count similar to 3, 4, 5 of the modification Quigley-Hein Plaque Index [10] and high gingival index (count similar to 2, 3 modification Gingival Index of the Lo e-Silness [11]. These evaluations had been reiterated following forty-five and ninety days of usage of the mouthwash, respectively. Participants were told to clean the teeth as normal and to rinse with 10 mL of MGP 5% for a minute following their morning and night meals. During the trial, subjects were not permitted to use any other kind of mouthwash. Once fresh supplies were distributed, participants were requested to send back their old ones so that compliance with products could be tracked.
Reproducibility of Clinical Examinations
All assessments were done by a single examiner who had been trained to maximize the research's consistency. Before beginning the investigation, the researcher taught the dental inspector as a "gold standard," instructing him to gently insert the periodontal probe into the gingival sulcus, keeping the instrument parallel to the long axis of the tooth, and gliding it from the distal to the mesial side of each assessed tooth. Nine participants who were not included in the study were evaluated for calibration. A conceptual calibration was carried out for the plaque index. Following shortly thereafter, pictures were employed to obtain an intra-examiner standardization.
The photographs were shown to the examiner by the adviser, who noted the plaque index values for every image. Following 15 days, the identical images were shown to the examiner, who recorded the plaque index values repeated. The plaque index acquired both the initial and second times was compared in order to confirm the intra-examiner value. Afterwards we achieved a kappa value of 0.73, which was deemed a significant estimation of reliability [14].
Data Analysis
In the present investigation, data were analyzed using the statistical program SPSS Version 21. For getting a nonparametric distribution, the mean modified in the starting point for both scores of the modified Plaque Index of Quigley-Hein [10] and modified Gingival Index of Lo e-Silness [11] as well as the equivalent severity scores were contrasted via covariance analysis, by Friedman test, for data obtained at 45 and 90 days of the research. All statistical hypotheses tests had two strands, and a significance level of P.05 was used [15,16].
RESULTS
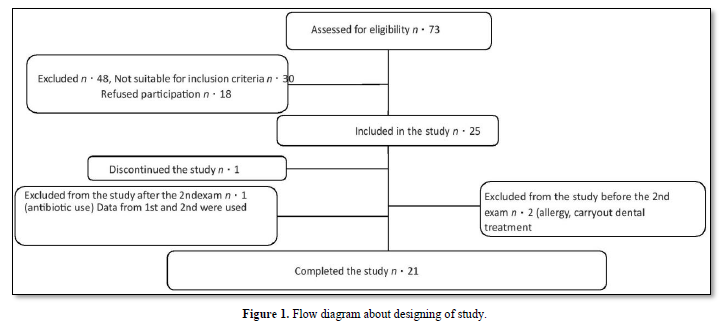
Throughout the time accessible to the research's development, a convenience selection was conducted, with 73 patients being qualified. Due to the inclusion and exclusion criteria and access, just one representative sample of 25 persons could be included, which is consistent with what was suggested [17,18] for phase II studies (Figure 1). The research team included twenty-one people (10 males and 11 females). Others departed from the research for a variety of reasons, including one who sought dental treatment throughout the research and was eliminated; second who had a suspected allergy to propolis; one who left due to having felt hyper sensibility on teeth; and one who was excluded for taking antibiotics owing to illness. This recipient's data was incorporated in the 45-day analysis.
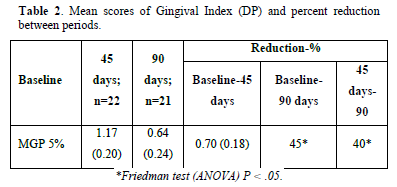
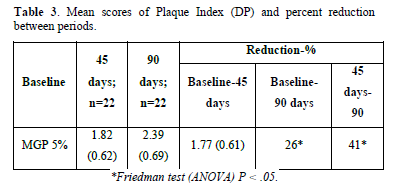
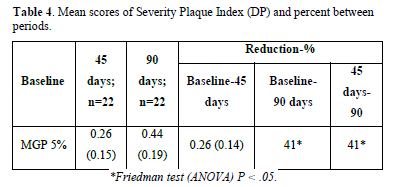
Gingivitis and Gingival Severity: The mean baseline Gingival Index GI scores at 45 and 90 days were reported. The MGP 5% revealed a decrease in gingivitis of more than 40%, which was statistically significant when compared to the baseline scores of 45 and 90 days (P.05). However, there was no statistically significant reduction in gingivitis when contrasting 90-day to 45-day scores (Table 2). The MGP 5% reduced the rate of the surface with plaque index scores of 3, 4, and 5. When comparing the mean baseline scores with 45 and 90 days (P.05), the decrease was statistically significant (more than 70%). There wasn't no statistical significance when the mean scores of 45 and 90 days were compared (Tables 3 & 4).
Oral Mucosa Changes: The existence of an exophytic lesion in the free marginal gum of the labial surface of element 27 was noted in only one patient during the previous dental examination done by the investigator. This lesion had a reddish surface, was smooth and bled when touched, and measured 2 2 mm. Plaque buildup was seen surrounding the lesion. Considering these findings, pyogenic granuloma was proposed as a probable diagnosis. Following that, a supra- and subgingival scaling was performed in this lesion location, leading to its total regression in 15 days. During the three months of treatment, one patient experienced a burning sensation in the oral mucosa for a brief amount of time after using the mouth rinse. Another 3 patients reported that during the period using mouth rinse they had a dryness sensation in the mouth.
DISCUSSION
This phase II trial study evaluated the action of MGP 5% on gingivitis and periodonto-pathogenic plaque. Clinical trials often present limitations independent on the efforts of the researchers.
The present research had limitations, including the occurrence of unforeseen product events and a possible allergic reaction that did not warrant caution but reduced the sample size by excluding one person. Another obstacle was the challenge of monitoring research compliance and contacting participants each time they required to return for assessment. Clinical trials contain limits with regard to the authenticity of the product's acceptability by patients, factors that are typically outside of the control of the investigator, regardless the implementation of a controlled-use mouthwash (return the empty bottle). As weighed against baseline scores index at 45 and 90 days, the MGP 5% produced substantial decreases in supragingival plaque and gingivitis as an adjuvant to the dental hygiene routines. The results are most likely supported by propolis's antibacterial and anti-inflammatory properties. Dental plaque's bulk decreased as a consequence of a decrease in bacteria. Propolis, in a variety of formulations, has shown effectiveness against periodontal infections in certain in vitro and in vivo tests [19-21].
Flavonoids, phenolic acids, and their prenylated derivatives are found in Brazilian propolis, which is why it has antibacterial properties. Considering the type of bee that created it, its place of origin, and the seasons of collecting, propolis has a complicated chemical composition. In the present investigation, we considered the time of use, evaluation times, and concentration of the mouthwash because its action is dose-time dependent. Caffeic acid, benzoic acid, and cinnamic acid are among substances found in propolis that may act on the microbial membrane or surface of the cell wall, causing structural and functional damage [22]. These substances also include flavonoids (quercetin, galangin, and pinocembrin).
While it is widely known that a single propolis component does not have an activity that exceeds the rest of the elements of propolis separated, synergistic actions of several compounds appear to be the most significant mechanism that defines the antibacterial activity of propolis [19]. The green propolis from the native Brazilian plant Baccharis dracunculifolia was employed in this investigation [23]. The artepillin C and other chemicals like coumarinic acids, which are likely associated to anti-inflammatory and antibacterial characteristics, respectively, are the principal bioactive components of this type of propolis. Additional research on the artepillin C compound suggested that it may have anti-inflammatory properties [24-26]. Other substances found in green propolis could be responsible for the anti-inflammatory properties seen in its research. Propolis modulates cytokines and inflammatory enzymes to provide an anti-inflammatory effect, including by reducing the formation of prostaglandins, leukotrienes, histamine, and TGF-1 [26,27]. By reducing the amount of bacteria in dental plaque, the byproducts they release-which serve as a trigger for gingival inflammation-decrease as well. The effects of MGP 5% on gingival and plaque severity show that the anti-inflammatory effect on individuals' gingival health was stronger than the effect of lowering plaque, as a consequence of a greater reduction in bleeding points than a decline in plaque.
Due to the fact that gingivitis is a chronic condition and that the study participants' hygiene practices were unaffected, a 3-month treatment term was selected. In this investigation, we used a methodology that was very similar to the requirements demanded by the ADA for testing new items intended for use in the oral cavity. The overall goal of such goods and approaches, according to the ADA, is to make it easier to identify areas or people who already have periodontitis or who are more likely to acquire it in the future. Clinical use of such diagnostics might occur (1) during initial evaluation (screening, pretreatment risk assessment, diagnosis, and treatment planning); (2) during treatment or management (monitoring therapeutic endpoints and identifying therapeutic targets), and (3) posttreatment (establishment of recall intervals and early detection of recurrent disease). The specifics of clinical trial design for each of these related, but separate, clinical functions may differ depending on a variety of circumstances [28].
As a phase II study, time commitment is not a critical necessity [29,30]. The concentration of 5% was chosen since propolis is a resinous substance and, at larger percentages, could precipitate in the bottle, staining the teeth and making patients uneasy owing to the strong flavor. There haven't been any research investigations up to this point that assessed the propolis oral rinse's ability to prevent plaque and gingivitis for as long as we did [29,31,32]. Following the baseline investigation, oral screening was conducted throughout the three-month research period. One patient had a pyogenic granuloma in the buccal marginal gum of element 27. The occurrence of plaque buildup in this tooth indicates that the individual concerned did not maintain proper oral hygiene, which was confirmed by an oral examination. This theory is supported by the scaling-induced plaque elimination, supra- and subgingival plaque, and the lesion's regression following 15 days [33]. Those people who complained of dry mouth and rough presumably felt that way because they weren't getting sufficient fluids to hydrate their bodies. Additionally, these patients were female and, in the age, range associated with menopause. The sensation vanished after the patient began to consume the necessary amount of water. Despite the fact that the MGP 5% contains no alcohol, one patient said that after using the mouthwash for three months, they experienced burning. This was likely owing to the mouthwash's 5% concentration and strong flavor, given none of the other respondents had mentioned this. This patient had no mucosal inflammation upon intraoral inspection.
The need for alcohol-free mouthwashes has increased recently for a number of factors. It was originally decided not to include alcohol in the mouthwash for social and health concerns. Religious restrictions and the possibility of smelling alcohol on the breath are examples of social justifications. Furthermore, it is known that some people's oral mucosa is sensitive to alcohol, and there is some evidence to support the idea that discomfort rises linearly with rising alcohol concentrations. The weakening and decreased color stability of tooth-colored restorations as well as a potential rise in the risk of oral cancer are other issues that alcohol rinses may cause [34].
CONCLUSION
The results of the current study demonstrated the effectiveness of alcohol-free mouthwash comprising 5% Brazilian green propolis in preventing and treating gingivitis and plaque, indicating that it may be utilized as a therapeutic and preventative measure to manage periodontal disorders. Nevertheless, before the use of an alcohol-free mouthwash that contained 5% w/v of Brazilian green propolis becomes standard in dentistry, it is essential to conduct a clinical trial that is double-blind, randomized, and adheres to the standards of the Council on Dental Therapeutics of the American Dental Association (ADA).
No Files Found
Share Your Publication :