-
Publish Your Research/Review Articles in our High Quality Journal for just USD $99*+Taxes( *T&C Apply)
Offer Ends On
Michel Goldberg*, Victor Arana-Chavez E and Chen Zhi
Corresponding Author: Michel Goldberg, Department of Oral Biology, Faculty of Fundamental and Biomedical Sciences, INSERM UMR-S1124, Paris Cité University, France.
Received: March 14, 2023 ; Revised: March 18, 2023 ; Accepted: March 21, 2023 ; Available Online: March 31, 2023
Citation: Goldberg M, Arana-Chavez VE & Zhi C. (2023) The Periodontal Ligament, Link Between the Root and Alveolar Bone. J Oral Health Dent Res, 3(1): 1-9.
Copyrights: ©2023 Goldberg M, Arana-Chavez VE & Zhi C. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Views & Citations
Likes & Shares
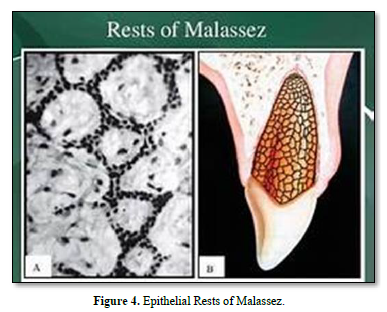
The periodontal ligament (PDL) connects the root and alveolar bone. Anchored to mineralized surfaces, it includes collagens and oxytalan fibers. Metalloproteinases play role in the turnover of type I collagen and extracellular matrix proteins. After dissociation of the epithelial sheath, epithelial rests of Malassez constitute a source of stem cells. Osteonectin and biglycan were detected in PDL cells. The levels of the receptor activator of NFkB ligand (RANKL) and osteoprotegerin (OPG) implicate changes of amount of RANKL, OPG, matrix proteins (collagen, fibronectin, SPARC, glycosaminoglycans (GAGs) and proteoglycans (PGs), MMPs and TIMP - 1 to 4). We explore here 1) the attachment function of the PDL (eruption, formation and supportive functions), 2) the implication of stem cells in healing, regulation of bone volume, and PDL regeneration, 3) the contribution of fibroblast-like and pericytes to periodontal inflammation, 4) sensory events (Ruffini-like mechanoreceptors), 5) vascular supply, and 6) because the cells of the PDL differentiate into cementoblasts and osteoblasts, this phenotypic availability contributes to heal the tooth supporting tissues. This structure initiates a process of self-organization resulting in a functional architecture of the PDL.
Keywords: Periodontal ligament, Radicular tooth, Alveolar bone, Cementum, Collagen fibers, Oxytalan fibers, Metalloproteinases, Epithelial rest of malassez
INTRODUCTION
The periodontal ligament acts as an attachment between the bone and dental root(s), including mostly collagens and oxytalan fibers. Many terms have been used to describe the periodontal ligament, including desmodont, gomphosis, peri-cementum, dental periosteum, alveolo-dental ligament and periodontal membrane. The PDL is anisotropic, heterogeneous, viscoelastic and possesses non-linear elastic properties. The effects of the PDL are restricted to the maxillary and mandibular alveolar bone [1-4].
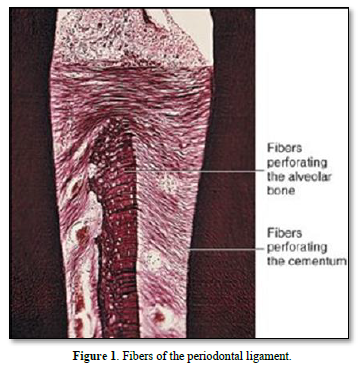
The periodontal ligament is thinner in the middle of the root and wider near the root apex. The “normal” width of the ligament (mean 25mm) is decreasing with age [5-8]. The functions of the ligament implicate nutrition, and multiaxial loading. The periodontal ligament consists of fibers coming from the pulp and gingiva, from the cribliform plate, from the vascular network, and from the nerves supply. Alveolar bone and cement are mineralized whereas the periodontal tissue includes the ligament and the gingival lamina propria. The PDL is composed by clastic cells, loose connective tissue (collagens and oxytalan fibers), fibroblasts and cell rests of Malassez [9] (Figure 1).
Characterization of periodontal ligament cells in vitro [10-11]
Alveolar bone can be divided into two main parts
In addition to the two layers, dense compact and cancellous bones form another layer, lining the alveolar socket. Blood vessels and nerves perforate the inner cribliform plate.
Periodontal ligament comprises five different groups of fibers mostly formed by fibroblasts:
The principal fibers ended from part and other in the cementum and in bone (named in such case Sharpey fibers) (Figure 2).
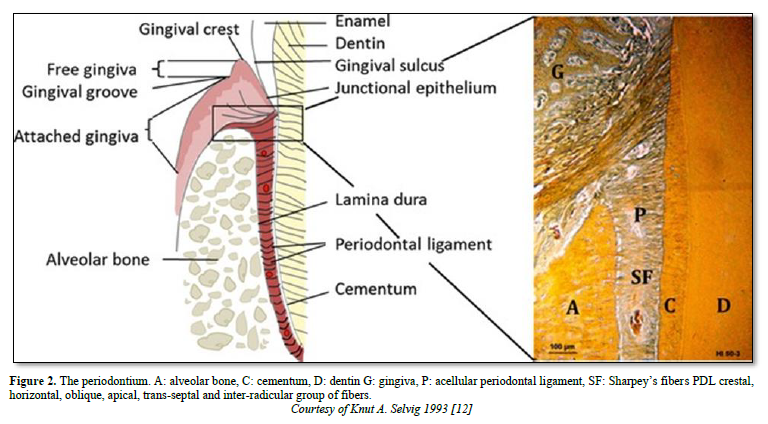
Composition of the periodontal ligament
Functions
Formative function plays a critical role in periodontal repair and regeneration, with high turnover rate of the PDL collagen.
Nutritive function.
Proprioceptive function.
Supportive function inside the bonny socket.
Homeostatic function.
The PDL is formed by:
Collagens
The collagen containing connective tissue includes type I, III, and VI and Type XII collagens (53-57%), embedded in the intercellular substance. Collagen fibers have roughly 55 nm in diameter (0,15 to 0,38 mm) [6-10]. Water has been estimated to be 70% (Figure 3).
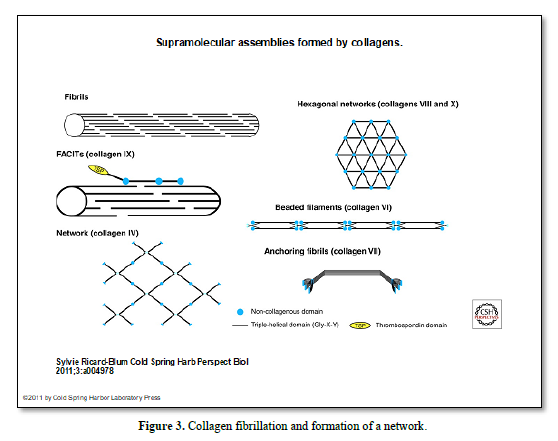
Collagen type XIV is expressed in the PDL, forming bundles 5 micrometers in diameter.
In vertebrates, the integrin family is composed of 18 subunits and 8 subunits that may assemble into 24 different heterodimers.
PA-TCH-SP and Con A staining of carbohydrates are very useful in identifying oxytalan fibers at the ultrastructural level [15]. They consist of bundles of filaments approximately 150 Å in diameter with an interfilamentous substance of the same diameter.
Cells of the PDL
Mensuration of the PDL The “normal” width of the ligament (mean 25mm) is decreasing with age. The thinnest part of the PDL is located in the middle third of the root. It increases near the apex.
Periodontal Ligament composition [22-23]:
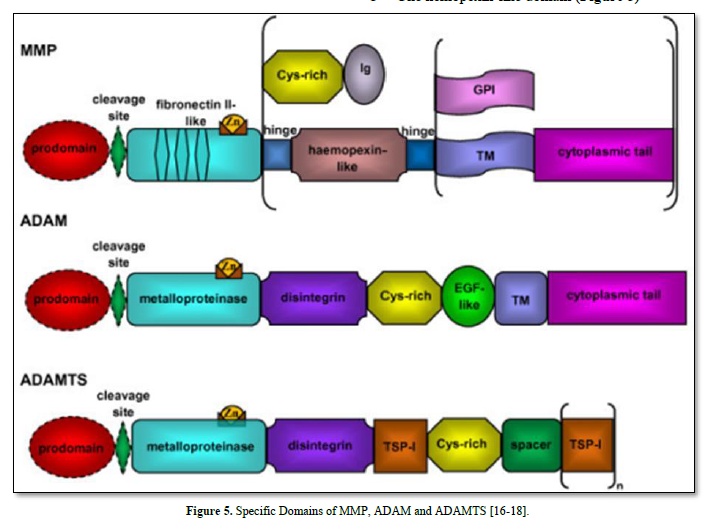
Innervation
Branches of the Vth cranio-facial nerve arises from the trigeminal nerve through its superior or inferior alveolar branches. The average density of myelinated nerve fibers increased by arriving closer to the apex. The diameter of myelinated fibers varied between 5.3 and 7.8 μm. The innervation is close to the alveolar bone. Isolated myelinated axons showed a tendency to group around large blood vessels. Dense innervations by myelinated nerve fibers reveals that apical as well as mesial and buccal sites are more densely innervated [11].
Type I receptor received its blood supply by diffusion whereas Type II receptor had a direct blood supply. Under each region Ruffini terminals and smaller terminals resembling free nerve endings were observed. The Ruffini terminals were unencapsulated and the majority had diameters of 2-3 microns. The terminals were observed near the junction of the inner and middle zones of the periodontal ligament with the axons running from the alveolar aspect. Periodontal mechanoreceptors, even those with more rapidly adapting properties, are Ruffini terminals.
Type II mechanoreceptors are the primary mechanoreceptors in the periodontal ligament. The periodontal Ruffini endings display dendritic ramifications with expanded terminal buttons and furthermore, are ultrastructurally characterized by expanded axon terminals filled with many mitochondria and by an association with terminal or lamellar Schwann cells.
Corpuscles of Ruffini are present in the PDL, forming a peripheral innervation. The blood capillaries showed a continuous endothelium. They are showing little adaptation capacities. They respond to sustained pressure and they are slowly adapting receptors.
Histochemically, the axon terminals are reactive for cytochrome oxidase activity, and the terminal Schwann cells have both non-specific cholinesterase and acid phosphatase activity. It has been suggested that the Ruffini endings have a high potential for neuroplasticity. Immunoreactivity for p75-NGFR (low-affinity nerve growth factor receptor) and GAP-43 (growth-associated protein-43), both of which play important roles in nerve regeneration/development processes, have been reported in the periodontal Ruffini endings. In experimental studies on nerve injury to the inferior alveolar nerve, the degeneration of Ruffini endings takes place immediately after nerve injury, with regeneration beginning from 3 to 5 days later, and the distribution and terminal morphology returning to almost normal at around 14 days. During regeneration, some regenerating Ruffini endings expressed neuropeptide Y. On the other hand, the periodontal Ruffini endings show stage-specific configurations which are closely related to tooth eruption, suggesting that mechanical stimuli due to tooth eruption and occlusion are a prerequisite for the differentiation and maturation of the periodontal Ruffini endings.
Numerous myelinated and non-myelinated nerve fibrils, as well as nerve endings and mechano-receptors are identified in the PDL [23]. Using a PGP 9.5 antibody staining it was shown that the apical region was richly supplied with nerve terminals. Neurofilament protein (NFP) and glia-specific S-100 protein., released by sympathetic nerves have been identified in the ligament, whereas there is no evidence of a parasympathetic innervation in the PDL [22-24].
Vascular supply
Vascularization arises from the superior and inferior alveolar arteries, derived from a series of perforation arteries that pass through the alveolar bone, and occupy interstitial spaces. Many arteriovenous anastomoses occur within the PDL. Lymphatic vessels tend to follow the venous drainage. The abundance of microfilaments and microtubules as well as the presence of intermediary and tight junctions characterize the vascular supply.
Vascular specific markers CD31 and vascular endothelial growth factor (VEGFA) are highly expressed in the PDL. SMAD 3, integrins and VEGF have increased expression in the PDL during orthodontic tooth movement. Nexus between the cell processes indicate that these cells are contractile and motile. The vascular distribution is asymmetric: vascularization is seen to be adjacent to bone, but not to cementum [25].
Arteries enter the PDL space from the alveolar bone. They are referred to as perforating arteries. Dental inter-septal artery enters the PDL space and contact anastomosis with gingiva blood vessels. They are referred to as “hydraulic pressure distribution” or “functional regeneration and adaptation”. Neuropeptides are released and fenestrations allows nutriments to diffuse in the PDL. The veins drain into the interdental veins or into the periapical plexus.
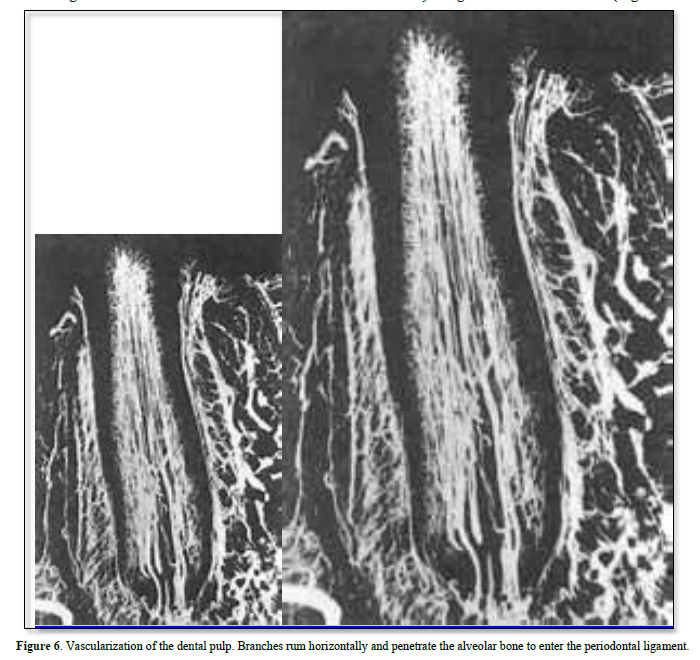
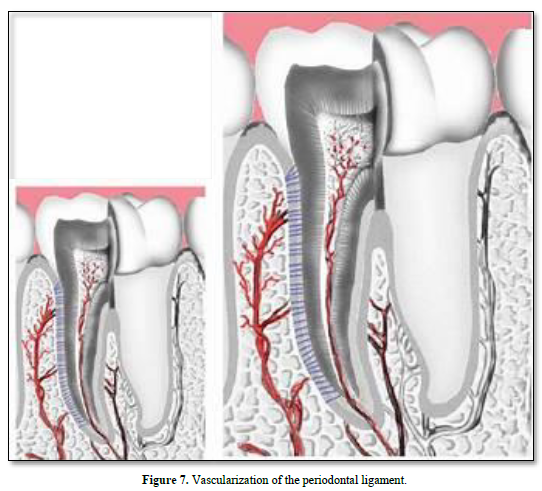
The lymphatic vessels drain into the regional lymph nodes, and finally merge with the thoracic duct (Figures 6 & 7).
CONCLUSION
The periodontal ligament constitutes a physical link between the cementum and bone of the alveolar crest. In addition to the PDL functions as support, sensory, nutritive, and remodeling, an attachment is induced to the inner bone socket. Epithelial Hertwig epithelial rests may provide stem cells, implicated in the healing and regeneration of the PDL. Tissue repair of the ligament, and sensory events are requiring nerve corpuscles (namely Ruffini endings) and vascularization. PDL include collagen and oxytalan fibers, phosphorylated and non-phosphorylated molecules, blood serum derived alpha2-HS-glycoprotein, fibronectin, glycosaminoglycans and proteoglycans, a number of extracellular matrix proteins (including fibronectin, and SPARC proteins), metalloproteinases and tissue inhibitors of MMPs [26]. This heterogeneous composition shed lights on the complexity of this structure and to the diversity of functions.
It is concluded that PDL has 1) an attachment function to bone and cementum (eruption, formation and supportive functions), 2) STEM cells are implicated in healing. The PDL is involved in the regulation of bone volume, PDL repair, tissue homeostasis and regeneration, 3) fibroblast-like and pericytes cells of the PDL contribute to periodontal inflammation. Hence, the cells of the PDL contribute and release molecules implicated in periodontal inflammation, 4) they are also implicated in sensory events requiring nerve corpuscles, and providing vascular supply and nutrients to the cementum, alveolar bone and to the PDL itself. 5) because the cells of the PDL may differentiate into cementoblasts and osteoblasts their phenotypic availability contributes to the healing and regeneration of tooth supporting tissues [27].
No Files Found
Share Your Publication :