-
Publish Your Research/Review Articles in our High Quality Journal for just USD $99*+Taxes( *T&C Apply)
Offer Ends On
Michel Goldberg*, Chen Zhi and Victor Arana-Chavez
Corresponding Author: Michel Goldberg, Department of Oral Biology, Faculty of Fundamental and Biomedical Sciences, INSERM UMR-S1124, Paris Cité University, France.
Received: March 08, 2023 ; Revised: March 11, 2023 ; Accepted: March 14, 2023 ; Available Online: March 31, 2023
Citation: Goldberg M, Zhi C & Arana-Chavez V. (2023) Indirect and Direct Pulp Capping, Apexogenesis and Apexification. J Oral Health Dent Res, 3(1): 1-11.
Copyrights: ©2023 Goldberg M, Zhi C & Arana-Chavez V. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Views & Citations
Likes & Shares
Pulp therapies aiming to keep alive the dental pulp use indirect or direct procedures. It is dependent on the stage and depth of the carious lesion, on the exposure time, on the degree of bacterial invasion, associated or not with pulp degradation. Our first aim was to clarify the formation of reactionary or reparative dentins, followed by pulp regeneration. Apexogenesis is a phenomenon implicating a vital pulp. Root lengthening is associated with tooth eruption. The treatment objective is to maximize the opportunity for apical development and closure. Apexification is occurring when the pulp is non-vital, infected or not. Apexification enhanced the continued root dentin formation inside the lumen, linked with apical closure and possibly with radicular dental pulp regeneration. A new treatment option of revascularization has recently been introduced after triple antibiotic therapy disinfecting the root canal system, laceration of the periapex with a file until bleeding occurs, providing a matrix of blood clot into which cells could grow. It involves also the sealing of the coronal access. Root end closure is what is referred to apexification, associated or not with pulp regeneration.
Keywords: Indirect pulp capping, Direct pulp capping, Reactionary dentin, Reparative dentin, Apexogenesis, apexification, Caries, Bioactive capping agents, Pulp regeneration
Abbreviations: Ca(OH)2: Calcium Hydroxide; MTA: Mineral Trioxide Aggregate
INDIRECT AND DIRECT PULP CAPPING: INTRODUCTION AND GENERALITIES
Indirect pulp treatment
The most appropriate treatment of the carious teeth implicates primarily to make a good diagnosis of the pulpal status. Spontaneous severe pain is a crucial factor. Finger pressures, radiographs, unrestorable tooth after pulp therapy, and teeth close to exfoliation are limiting factors to indirect pulp capping.
The stepwise excavation approach intends to change the carious environment and not to remove the carious tissue close to the pulp taking into consideration the risk of pulp exposure. The aims of this technique is:
The rationale for this treatment option implies to encourage the formation of a reactionary layer of dentin with preservation of pulpal health and vitality. This implicates the removal of the deep carious lesion, and restoration of the crown after the placement of an appropriate material (firstly lining the deepest part of the lesion and secondly, restoring the cavity with an appropriate biomaterial). About 90% clinical success was observed after 3 years follow up. This procedure is recommended for permanent teeth but not for primary molars.
Indirect pulp capping implicates the preservation of a layer of soft carious dentin. Removal step-by step with hand instruments (excavators) of the carious dentin layers suggests to keep some demineralized dentin in the deep part of the deteriorated tissue. It is mandatory to avoid drilling with rotating burrs. Elimination of the carious dentin, associated with substantial changes in preventive measures: hygiene conduct, and limitations in carbohydrates intake favor remineralization. With indirect capping, there is an 86% rate of success over 10 years.
Beneath a dense calcio-traumatic line, tubular or atubular reactionary dentin (or tertiary dentin) is formed. This tertiary dentin is developed in reaction to transdentinal stimulation of the odontoblasts, bacterial noxious or cytotoxic components, some being released by restorative materials. Reactionary dentine may be considered as an extension of physiological dentinogenesis. However, since it is a pathological response to injury, it is distinct from the primary and secondary dentinogenesis.
Removal of the soft carious dentin, covered by a temporary material containing a calcium hydroxide base, is beneficial for the evolution of active caries into an arrested lesion. Zinc oxide-eugenol cement is laid down, and after 2-3 weeks, the arrested carious dentin (sclerotic or sound) is actually removed, and a “permanent” filling may be inserted. The risk of pulp exposure is increased by the complete excavation of caries compared with stepwise excavation and/or indirect pulp capping [2].
The biologic validity of the various vital pulp treatments involves indirect pulp treatment. It is an acceptable procedure for primary teeth with reversible pulp inflammation. Indirect Pulp Treatment (IPT) was a success in 95% carious lesions. Calcium hydroxide liners increased the success rate of IPT. Direct pulp capping (DPC) and calcium hydroxide has been widely used with high success rates in young permanent teeth, but the results in primary teeth are less satisfactory [3,4]. The rationale for using calcium hydroxide should be reserved for iatrogenic exposures in asymptomatic teeth expected to exfoliate within a short period of time. Indirect pulp treatment is recommended for teeth that have deep carious lesions approximating the dental pulp, but no signs or symptoms of degeneration. Indirect pulp treatment, using calcium hydroxide as liner, gives after 2 years 83% of success. In this procedure, the deepest layer of the remaining carious dentine is covered by a biocompatible material to prevent pulp exposure. Three materials are most commonly used in indirect pulp treatment: the calcium hydroxide (CH), zinc oxide-eugenol paste (ZOE) and glass ionomer cements. There is high probability that this solubilizing effect of cavity conditioners results in the release of TGF from the tissue, diffusing through the dentinal tubules, promoting a reactionary dentinogenic response stimulated by the underlying odontoblasts.
The ultimate objective of this treatment is to maintain pulp vitality, while promoting dentin sclerosis and carious (reducing permeability), stimulating the formation of tertiary dentin, and remineralization of the carious dentin.
Pulp healing is influenced by the prevention of bacterial leakage. Operative debris, inflammatory and pulp cell activity, absence of dentin bridge formation, tunnel defects in dentin bridges emphasized the failures of direct capping.
Pulp exposure after stepwise removal of the bulk carious tissue is followed by the application of calcium hydroxide. After a period of 8-24 weeks, all the carious dentin was removed, following sealing of the cavity with ZOE and a restorative material [5]. The control of pulp bleeding after exposure has a direct effect on pulp cap success. Saline sodium hypochlorite (at concentrations ranging from 0.12% to 5.25%), hydrogen peroxide, ferric sulfate and chlorhexidine have shown their usefulness. The chances for tooth survival are excellent if the tooth is asymptomatic and well-sealed, even if residual caries remains [6]. MTA demonstrates comparable results to calcium hydroxide. MTA demonstrates successes as a direct pulp cap agent in short-term data.
When a healthy pulp is inadvertently exposed during an operative procedure, a calcium hydroxide [Ca(OH)2] may be placed over the exposure and dentine formation is stimulated. Direct pulp capping by calcium hydroxide is associated with dentin bridge formation. Tunnel defects are present in the area of operative debris, together with the pulp inflammatory activity. Direct pulp capping is one of the treatments of an exposed vital pulp. It is used to stimulate the maintenance of a vital pulp. It is however mandatory to develop new biologically-based therapeutics that reduce pulp inflammation, promote the continued formation of a renewed dentin-pulp complex, and restore vitality by stimulating the regrowth of pulpal tissue. The odontoblast-like cells secrete tertiary dentin (reparative dentin) either when irritated by the chemicals diffusing through the dentin, or when toxic bacterial metabolites diffuse through dentinal tubules.
The capping materials were associated with varying levels of pulp healing defects, including tunnel defects, operative debris, pulp inflammatory cell activity and bacterial leakage [7]. TGF and Bone Morphogenetic Proteins may also induce reparative dentinogenesis in a direct pulp capping situation. The ideal dressing material for the radicular pulp should be bactericidal, harmless to the pulp and surrounding structures, promote healing of the radicular pulp, and not interfere with the physiologic process of root resorption. Recent clinical studies have reported promising results using ferric sulphate (FS), a hemostatic agent, in pulpotomized human primary teeth. Better results were obtained with Mineral Trioxide Aggregate (MTA), and statistically significant differences were reported when compared with ferric sulphate. Success was defined as lack of complaints from the patient, positive reaction to cold testing, and no sensitivity to percussion [8].
The aim of vital pulp therapy is to maintain pulp viability by eliminating bacteria from the dentin-pulp complex and to establish an environment in which apexogenesis can occur. A complicating factor in treating immature teeth is the difficulty to predict the degree of pulpal damage. Currently, the best method appears to be the ability to control pulpal hemorrhage by using sodium hypochlorite. Mineral trioxide aggregate (MTA) currently is the optimum material to use in vital pulp therapy. Compared with the traditional material of calcium hydroxide, it has superior long-term sealing ability and stimulates a higher quality and greater amount of reparative dentin. The control of pulpal hemorrhage with NaOCl seems to be the best method of treatment, MTA being a good substitute for Ca(OH)2 in vital pulp procedures.
The successes of direct pulp capping are less, decreasing to 37% after 5 years, and 13% after 10 years. Direct capping is effective when it is carried out on a pulp exposure, revealing an initial opening of a pulp horn, and accompanied with bacterial invasion. The exposed coronal pulp leads to treatment of the pulp by direct capping, using calcium hydroxide or others bioactive drugs. The formation of reparative dentin, occluding the pulp exposure produce a bone-like structure, made of collagen and non-collagenous proteins (namely osteocalcin and osteopontin). This bonny structure is called osteodentin.
Reparative dentinogenesis represents a more complex sequence of biological processes. Migration and differentiation of pulpal progenitor cells must take place, followed by a generation of odontoblast-like cells. A series of stereotypic wound healing reactions occur in the pulpal connective tissue, that includes vascular and cellular inflammatory reactions. In vitro and in vivo experiments on reparative odontogenesis demonstrate that the pulp constitutes an appropriate environment where competent pulp cells that are potential pre-odontoblasts differentiate into new odontoblast-like cells, forming dentin occluding the pulp exposure.
TREATMENT OF PRIMARY TEETH
The remaining radicular pulp can be rendered inert by using formocresol. It fixes or denatures the vital pulp, so there is no longer a pulp that is alive. The radicular pulp might be preserved through minimal inflammatory insult by using a hemostatic agent such as ferric sulfate to preserve the deeper remaining pulp tissue. Pulpotomy mechanism encourages the radicular pulp to heal and form a dentin bridge by using calcium hydroxide or mineral trioxide aggregate [4].
Practitioners should not try to have access to the sclerotic carious layers, isolating the pulp from the advancing front of the lesion. This is why in addition to indirect pulp capping; the two stages procedure or stepwise excavation has been introduced in the everyday practice (Figure 1) [1]. For deep caries approaching the pulp, the choice of indirect pulp treatment (IPT) is up to the treating dentist. IPT has been shown to have a lower cost, higher long-term success, better exfoliation pattern treating reversible pulpitis instead of pulpotomy (Figure 2).
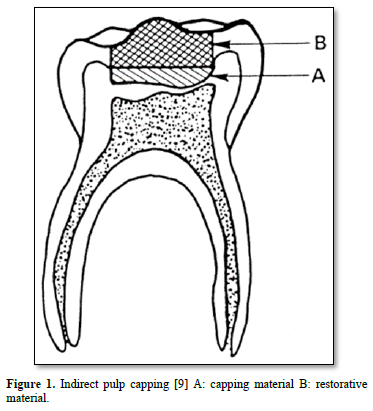
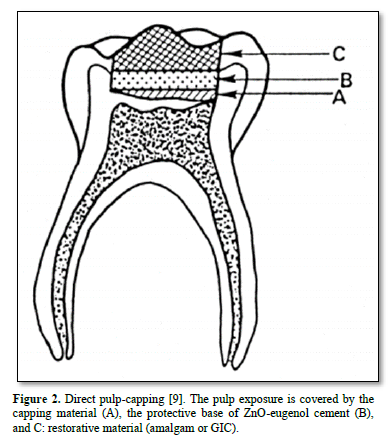
Treatment options: biomaterials used for indirect and direct capping
Calcium hydroxide (Ca (OH)2)
Since more than fifty years, a number of applications was made such as root resorption, intracanal medicament, perforation management, and root canal sealers. Ca(OH)2 is a strong base that dissociates with aqueous calcium hydroxide and hydroxyl ions [10]. After an initial bactericidal effect, it promotes coagulation necrosis occurring together with the formation of osteodentin [6].
Antibacterial activity was combined with chlorhexidine or sodium hypochlorite against Gram-positive and Gram-negative bacteria. Ca (OH)2 and NaOCl had effects on all the tested bacteria, while 2%CHX was less effective [11]. The antibacterial effect due to the destruction of the bacterial cytoplasmic membrane, is followed by protein lysis and bacterial DNA damage. Osteodentin formation was uncomplete and results in the formation of tunnel defects, allowing reinfection [12].
The effects caused by several sequential mechanisms:
Over the years, calcium hydroxide has been the most commonly employed wound dressing. It creates conditions conducive to healing of the pulp tissue. The outcome of MTA and calcium hydroxide treatment, with or without pulpal exposure, depends largely on how extensively the pulp is infected at the time of treatment. The outcome also depends on the age of the patient, the treatment approach (indirect or direct pulp capping) and the choice of material applied to the exposed pulp tissue. The capacity of the restorative material to prevent leakage of bacteria is another important factor [2].
The initial effect of calcium hydroxide applied to exposed pulp is the development of a superficial necrosis. Necrosis causes slight irritation and stimulates the pulp to repair. Direct pulp capping is occluding the pulp exposure by reparative dentin formation. Multiple tunnel defects were shown and cell inclusions in bridges following pulp capping with Ca(OH)2. Eighty-nine per cent of dentin bridges formed by calcium hydroxide cement contained tunnel defects. This may lead later to bacterial penetration into the pulp tissue. The tunnels are not caused by the Ca(OH)2 itself but are rather are a consequence of the severity of the trauma to the pulp and the number of vessels injured by the mechanical exposure. Inside the tunnels there are blood vessels, which maintain the calcium source to the necrotic tissue (coagulation necrosis). The calcium ions in the necrotic layer are responsible for partial dystrophic calcification. Cells in contact with Ca(OH)2 are killed, due to the alkaline pH, forming a necrotic layer of variable thickness. The success rates of direct pulp-capping with calcium hydroxide decrease with time. Rates are more than 90% after 1 to 2 years and drop from 82% to 37% after 2 to 5 years.
The subjacent pulp tissue is responsible for the healing associated with hard tissue barrier formation. The multiple tunnel defects present a morphological disruption of the dentin bridge barrier. They fail to provide a long-term biological seal against bacterial infection. Multiple defects in the bridge allow fluid and bacteria to penetrate into the pulp, which in turn can lead to internal resorption, and ultimately to tooth loss. Consequently, the tunnels permit oral contaminants, such as bacteria and their toxic factors, to eventually gain access to the pulp tissue through the marginal gap formed at the tooth / restoration interface. The presence of bacteria and their products that penetrate via microleakage is the main factor responsible for pulp inflammation and necrosis. Calcium hydroxide is still a «gold standard» for direct pulp capping materials Among other materials a number have been used with more or less success, all of them allowing to preserve pulp vitality.
Ferric sulphate has gained some popularity as a replacement for formocresol and calcium hydroxide in pulpotomies. It is claimed to have low toxicity and no systemic side effects.
Mineral trioxide aggregate (MTA)
MTA was used as direct pulp capping in young permanent teeth [13]. At 24 months the clinical and radiographic success rate was 93%, with some evidence of continued root growth.
Mineral trioxide aggregate (MTA) cement has a composition similar to that of 75% Portland cement (PC). In addition, MTA contains 20% bismuth oxide, which provides radiopacity. The major component is a mixture of dicalcium silicate, tricalcium silicate, tricalcium aluminate, gypsum, and tetracalcium aluminoferrite. MTA is a powder that contains trioxides and hydrophilic particles. It set in the presence of moisture. MTA cements exhibit calcified tissue-conductive activity and facilitate the differentiation of human orofacial mesenchymal stem cells and the mineralization process in human dental pulp cells. They also have the potential to be used as pulp-capping materials. MTA materials have been shown to have a biocompatible nature and have excellent potential when used in endodontic treatment. Thus, MTA can be described as a calcium-hydroxide releasing material and, therefore, is expected to present various properties similar to those described above for calcium hydroxide. The advantages of MTA are believed to be its sealing ability, biocompatibility, bioactivity and capacity to promote mineralized tissue formation.
In vitro bacterial leakage studies of MTA materials were found to exhibit similar root-end bacterial leakage resistance using a Streptococcus salivarius model with both materials having significantly less bacterial leakage than a ZOE preparation. Biocompatibility studies assess the compatibility by monitoring the expression of Interleukin [IL-1, IL-6, IL-8, IL-11] and macrophage colony stimulating factor (M-CSF). In vivo studies were shown to induce little or no inflammation. Pulp capping carried out on animal models or human studies have shown physical properties, sealing ability, biocompatibility, and clinical performance of MTA materials [14,15].
MTA is more effective and better than calcium hydroxide materials, as it has an enhanced interaction with dental pulp tissue. The pulp tissue necrosis is limited, facilitated the proliferation/differentiation of human dental pulp cells, and exhibited calcified tissue-conductive activity with the ability to stimulate faster complete dentin bridge formation and preventing infection.
Biodentine™
Biodentine™ contains mainly tricalcium and dicalcium silicate. Zirconium dioxide serves as contrast medium. The liquid consists of calcium chloride in aqueous solution mixed to polycarboxylate. Once mixed, Biodentine™ sets in approximately in 12 min. Calcium hydroxide is formed during the setting of the cement. It stimulates tertiary dentin formation. Calcium chloride accelerates the hydration reaction, and polycarboxylate reduces the amount of water required for mixing by providing proper consistency. Calcium carbonate in the powder is expected to act as a nucleation site. Biodentine™ was shown to be biocompatible, in that it does not damage pulpal cells in vitro or in vivo, and is capable of stimulating tertiary dentin formation. Used for pulp capping, the material offers certain advantages over calcium hydroxide [16]. The bioactive material Biodentine is stimulating apexogenesis, dentin replacement and pulp protection. A continued apical closure was detected on radiography. Direct pulp capping with MTA after pulp exposure maintain pulp vitality in permanent teeth [8].
TheraCal is a novel light- curable MTA-like material for pulp capping [17]. It is composed of resin and calcium silicate. TheraCal released significantly more calcium than ProRoot MTA and Dycal. The surrounding fluid was able to alkalinize. Initially the pH varies around 10-11, between 3 h and 3 days. Then the pH was decreased (8-8,5) between 7 to 14 days. Ca (OH)2-based and CaO-based materials were used for direct or indirect capping. They release hydroxyl and Ca ions. They are soluble and raise local pH with the formation of a necrotic layer at the material-pulp interface. This capping material has been designed for direct and indirect pulp-capping.
Calcicur (Voco) is a radiopaque water-based calcium hydroxide, containing urethane dimethacrylate resin, calcium dihydroxide, dimethylaminoethyl-methacrylate, and triethyleneglycol dimethacrylate (TEGDMA). Calcicur presented cytocompatibility values significantly lower than Biodentine (Septodont). Biodentine is an alternative to Ca(OH)2 versus MTA used for direct pulp capping [18], aiming to evaluate and compare the antimicrobial activity of different pulp-capping materials.
Antibacterial activity of Ca(OH)2 was combined with chlorhexidine or sodium hypochlorite against Gram-positive and Gram-negative bacteria. Ca(OH)2 and NaOCl had effects on all the tested bacteria while 2% CHX was less effective [11].
MTA-based products show lower cytotoxicity and valuable antibacterial activity. However, the conclusion that MTA-based pulp-capping material does not show cytotoxic effects in vitro should be taken with caution because the experimental design in vitro has some inevitable limitations with respect to the in vivo situation.
Although physical properties of resin composites are being improved constantly, in vivo studies have shown that the use of resins as restorative materials is occasionally associated with irritation and necrosis of the pulp. Most components of the adhesive systems and resin composites have been shown to have definite cytotoxicity when in direct contact with mammalian fibroblasts. Adhesive resin systems are used to enhance retention, reduce microleakage, and decrease postoperative sensitivity of composite resin restorations. Complete polymerization of adhesive resins might be unachievable during direct pulp capping procedures. In addition, it has been shown that the oxygen prevents complete polymerization of adhesive resin monomers. Consequently, unpolymerized monomers released from the resin-based material can diffuse directly into the pulp at the exposure site, as well as diffuse through the dentinal tubules to cause cytotoxic effects to the pulp cells. While unpolymerized and partially polymerized adhesive resin induced apoptosis were internalized rapidly in macrophages, undifferentiated pulp cells (OD) and mouse odontoblast-like cells (MDPC) incorporate polymerized adhesive resin and induced significant apoptosis only in macrophages. Bonding agents have been found to release a photo initiator and photosensitizer widely used to generate free radicals including reactive oxygen species.
Unfavorable effects of dentin bonding systems, pathological changes of pulpal tissues, such as dilatation and congestion of blood vessels, inflammatory responses and production of irregular dentin as well as odontoblastic displacement or tooth sensitivity occur after placement of composite restorations. The monomers in contact with oxygen are not converted into polymers. The persistent inflammatory reaction and hyaline alteration of extracellular matrix inhibit completely pulp repair or dentin bridging.
Apexogenesis
Apexogenesis takes in consideration the role that stem cells may have in the continued root development [22]. This is related to the biological activity of Hertwig’s epithelial root sheath (HERS). Stem cells have the capacity to self-replicate and differentiate into specialized tissue type. The odontoblasts and also at later stage, the pre-odontoblasts (Höhl cells) secrete dentin and are integral to the pulp - dentin complex. Primary dentin is formed until the full length of the root development is reached. This is followed by dentin formation that proceeds as secondary dentinogenesis and the associated tooth eruption. The outer- and inner-enamel epithelia constitute the two layers of the Hertwig’s sheath, a layer of cuboidal cells. They are also named Hertwig’s epithelial root sheath (HERS). This epithelial bilayer is derived from the inner and outer enamel epithelia. They fuse below the level of the cervical margin of the crown. These layers are widely accepted as the main region responsible for root formation. The results indicated that some stratum intermedium cells are originated from the inner enamel epithelium, while others are derived from the inner enamel epithelium. The cells of the Hertwig’s epithelial root sheath are linked by desmosomes and gap junctions, hemi-desmosomes being adherent to the inner- and outer- basement membranes. Mesenchymal cells of the dental follicle become cementoblasts. Cells from the dental sac cross the dissociated HERS. The outer layer disintegrates. Between the dissociated epithelial outer layer, the cells invade the surface of root dentin (the so-called “mantle dentin”). In contrast, acellular cementum and cementogenesis take origin from the inner epithelial cells. Bone-like molecular characteristics of cementum, and alkaline-phosphatase are essential enzymes to mediate cementogenesis.
Apexogenesis (Table 1) continues at a slower rate throughout the lifetime of the individual. As the root and pulp develop, the dental papilla located apically to the developing pulp contributes to the root formation. HERS is very sensitive to trauma and once destroyed by trauma or by infection, the normal root development is stopped without further differentiation of odontoblasts.
Formation of the apex
A series of dental tissues are formed in the apical zone. They include 1) the apical cell-rich zone, 2) the apical papilla mesenchyme and 3) the radicular dental pulp. Apexogenesis involves both lengthening (associated with eruption of the teeth), and the thickening of the dentin layer, to the detriment of the lumen (pulp space). Apexogenesis result from the contribution of cells issue from the dental papilla. They become after differentiation, odontoblasts involve in the production of dentin, whereas differentiation of cells from the cervical loop contribute to the lengthening of the tooth.
Apexogenesis is a natural physiologic process of root development. The term is used to describe the endodontic procedure of preservation of pulp vitality. It has also been suggested that maturogenesis is a more appropriate term than apexification, because not only the apex but the entire root is allowed to mature [19].
Calcium hydroxide induced the deposition of calcified material. It became the standard treatment protocol for the therapy of non-vital immature tooth. Many other biomaterials have been used to induce apexification, but none has replaced calcium hydroxide. Calcium hydroxide-induced apexification might require 6-24 months for barrier formation. The barrier formed is often porous and not continuous. Further development of the root does not take place. Intra-canal calcium hydroxide dressing can also make the tooth brittle because of its hygroscopic and has proteolytic properties. The rationale of revascularization is that if a sterile tissue matrix is provided in which new cells can grow, pulp vitality can be re-established. A matrix into which the cells from the periapical tissues could grow and re-establish pulp vascularity, may slowly replace the necrotic tissue.
It is possible that a few vital pulp cells remain alife at the apical end of the root canal. These cells may proliferate into the newly formed matrix and differentiate into odontoblasts. The newly formed odontoblasts can lay down atubular dentin at the apical end, causing apexogenesis, as well as on lateral aspects of dentinal walls of the root canal, reinforcing and strengthening the root.
The other possible mechanisms implicated in apexogenesis are the followings:
Complete root development requires a viable pulp containing cells that can differentiate into dentin-producing odontoblasts. The dental pulp is complex with a variety of cells, nerves, and blood vessels. It is important to keep in mind the required prerequisites, including
The procedure that induces apexogenesis is undertaken to preserve the remaining vital tissue and allow completion of root formation and apical maturation. Apexification is related to the immature teeth. Apexification is then performed to treat immature teeth with non-vital pulp by inducing a calcified barrier at the open apex [20- 22].
Apexogenesis is a natural physiologic process of root development. However, the term is used more commonly to describe the endodontic procedure of preservation of pulp vitality in a traumatized tooth with pulp involvement, so that the affected tooth could develop its full growth potential.
It was earlier unthinkable that the tissue in the periapical region of a nonvital infected tooth could regenerate.
A third possibility could be attributed to the stem cells from the apical papilla or the bone marrow.
Apexification
Pulp necrosis arrest further root development of an immature permanent tooth. To induce the formation of an apical mineralized tissue barrier, the method used specifically is named apexification. Because of its high pH, calcium hydroxide not only weakens the root but may also inhibit new tissue formation within the canal. The possibility of vital tissue regeneration in the root canal space with a continuous increase in root thickness and length has been demonstrated for immature teeth.
There are three major components in tissue engineering which are implicated in apexification:
Dental pulp stem cells are able to differentiate into functional odontoblast-like cells with an active mineralization potential. They may be used in dental tissue engineering via stem cell –based approaches. This is due to an increased alkaline phosphatase activity, dentine sialoprotein expression and to the formation of mineralized nodules. Platelet-rich plasma is a natural reservoir of various growth factors that can be collected, unlike the chemically processed molecules or recombinant proteins that may cause undesired side effects and expose the tissue to unnecessary risks. The use of platelet-rich plasma (PRP) in combination of DPCs may be beneficial for new tissue formation and for apical closure [22].
The protocol for pulp revascularization / revitalization begins with root canal irrigation with minimal instrumentation and then continues with disinfection with an antibiotic mixture. The most commonly reported dressing is a triple antibiotic paste (TAP), which consists of ciprofloxacin, metronidazole, and minocycline. It is presumed that the blood clot serves as a scaffold in which stem cells from the apical papilla (SCAP) populate the clot. In addition, growth factors released from platelets and the dentinal walls serve as a promoter for stem cell division and differentiation processes.
Immunohistochemistry and gene profile analysis have identified perivascular cells by using markers, such as alkaline phosphatase and smooth muscle actin, in differing proportions on STRO-1 positive cells. BMPs appear to be the key regulators of apexification.
The cell lines are grown and expanded before being implanted into the root canal, resulting in protracted clinical treatment times. The implanted cells then need to reliably adhere to the disinfected root canal walls. Lastly, the implanted tissue lacks a crucial vascular supply, and it is technically difficult to replant the three-dimensional regenerated pulp without damaging the cells. When an open root apex exists, a similar scaffold design adjacent to a vascular supply may assist apexification by thickening and closing the apical portion of the root with hard tissue.
No published reports are involving the use of genetically manipulated cells for apexogenesis or apexification procedures. Novel genes and finding appropriate vectors are mandatory to control cell-specific safe delivery. The phenotype repopulating the open root apex has still to be selected by environmental factors [23].
The apical part of the root includes three compartments: an apical cell-rich zone (where apical stem cells are mostly located and constitute a reservoir of undifferentiated pulp cells progenitors (SCAP cells- [24]. Cells are located in the apical papilla mesenchyme, and in the radicular dental pulp.
The goals of apexogenesis are the followings [24]
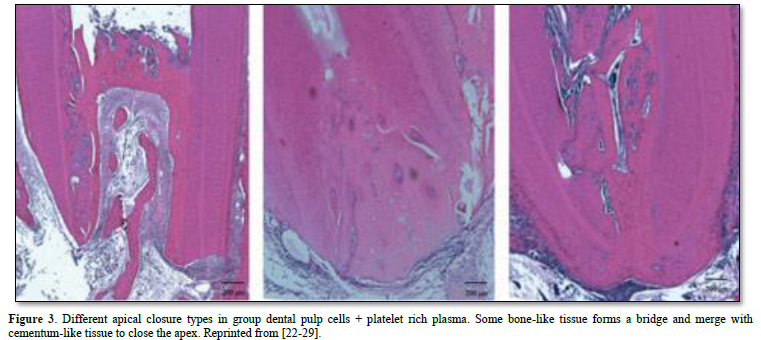
Apexification is a method of inducing a calcified apical barrier or continued apical development of an incompletely formed root in which the pulp is necrotic [26]. The developing consensus approach to accomplish apexification is to instrument root canals, to remove the necrotic tissue, and to place MTA in the root canal apex, with the remainder of the canal obturated with gutta-percha.
In the absence of a vital pulp, dentin deposition is arrested. When an immature tooth is affected by caries or trauma, the pulp requires proper management according to the degree of inflammation and keeping some vitality.
Artificial apical barriers are formed after implantation of a variety of materials. Apexification was demonstrated in a complete layer of cementum when using MTA as a root-end filling inducing apical hard tissue formation in immature roots.
An alternative treatment of the immature permanent tooth is apexification procedures. The classic apexification method involves long term application of Ca(OH)2. A more recent method of apexification involves the use of MTA as an apical barrier followed by placing either a root filling or obturating material. However, it is important to note that apexification by either Ca(OH)2 or MTA completely prevents any further root development in terms of increased radiographic measures of either root length or width. The immature tooth treated by apexification procedures demonstrates healing of apical periodontitis, but does not achieve the goals of continued root development or restoration of functional pulp tissue [27].
Apexification is defined as ‘a method to induce a calcified barrier in a root with an open apex or the continued apical development of an incomplete root in teeth with necrotic pulp’.
Success rates for calcium hydroxide apexification are high purification is a method of inducing apical closure through the formation of mineralized tissue in the apical pulp region of a non-vital tooth with an incompletely formed root. It is composed of osteocementum, osteodentin or bone, or by some combination of the three [28-30].
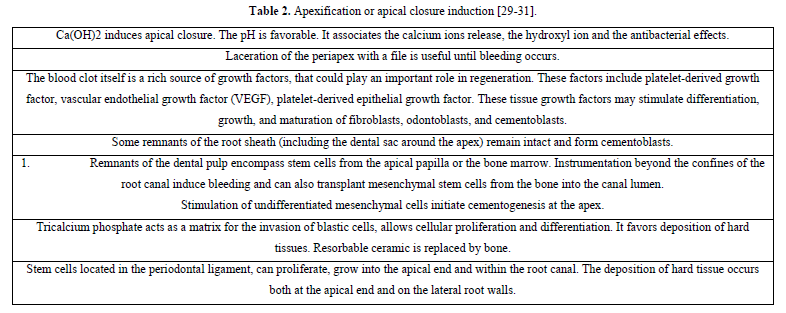
Apexification may be induced by Ca(OH)2 or MTA. Revascularization of necrotic pulp has been considered possible after traumatic injury to an immature tooth. A unique set of circumstances exist that favor revascularization. The potential for revascularization appears to directly depends on the race between bacterial infection of the necrotic pulp, and revascularization of the canal space using the ischemic pulp as a matrix [31]; Revascularization involves disinfection the root canal, providing a blood clot into which cells could grow and seal the coronal access. The canals were sampled before and after irrigation with 1.25% NaOCL and after dressing with a triple antibiotic paste, consisting of metronidazole, ciprofloxacin, and minocycline. The access cavity was sealed with a glass ionomer cement (GIC). An increased tooth length was observed. Revascularization procedures may be conducted on immature non-vital, infected permanent teeth [19]. An endodontic regeneration study on non-human primates by cleaning and shaping root canals. Then laceration of the periapical tissues cause bleeding into the root canals. If vital and not irreversibly inflamed, maintenance of vitality will allow natural continued root development. Maintenance of pulp vitality by using apexogenesis allow continued root development along the entire root length. Depending on the extent of inflammation, pulp capping, shallow or conventional pulpotomy may be indicated (Table 2). Traditionally, the approach has been to use calcium hydroxide (Ca(OH)2) to induce apexification after disinfection of the root canals.
Metronidazole is a nitroimidazole compound that exhibits a broad spectrum of activity against protozoa and anaerobic bacteria. It has been used both systemically and topically in the treatment of periodontal disease. Metronidazole readily permeates bacterial cell membranes. It binds to the DNA, disrupting its helical structure, and leads to rapid cell death. Completion of endodontic therapy was typically delayed until completion of root-end closure through apexification. Tetracyclines, which include doxycycline and minocycline. Tetracyclines are effective against most spirochetes, and many anaerobic and facultative bacteria. The tetracyclines gain access to bacterial cells. They act by inhibiting protein synthesis on the surfaces of ribosomes. Minocycline is a semi-synthetic derivative of tetracycline. It is available in many topical forms ranging from gel mixtures to sustained release microspheres. Ciprofloxacin, a synthetic floroquinolone, has a bactericidal mode of action. Ciprofloxacin has very potent activity against gram-negative pathogens but displays limited activity against gram-positive bacteria. Most anaerobic bacteria are resistant to ciprofloxacin. Side effects of ciprofloxacin have been reported. It was found that the drug is clinically safe when applied in low doses. When applied as an intra-canal medicament in low doses, adverse systemic side effects should be minimized.
Future directions
Bioactive pulp capping agents such as Calcium hydroxide, bone sialoprotein (BSP), bone morphogenetic protein (BMP-7 - also termed OP-1) were used successfully [19]. BMP belong to the superfamily of the Transforming Growth Factors. TGF of tissue repair in different situations. BMP-2, -4, and -7 play a role in the differentiation of adult pulp cells into odontoblasts during pulpal healing. Insulin-like growth factor-1 contributes to form a dentin bridge equal to Dycal after 28 days. Among Growth Factors, only the TGF-1 enhances reparative dentin formation. Enzymes such as Heme-Oxygenase-1, simvastatin, stem cells, Emdogain and ODAM have been used with variable success. Many of these molecules may constitute suitable replacement for calcium hydroxide.
Pulp healing include tunnel defects, operative debris, pulpal inflammatory cell activity and bacterial leakage. This is influenced by the nature and composition of the capping materials [23-33].
SUMMARY
A calcium phosphate material equipped with poly(lactic-co-glycolic acid) (PLGA) microspheres was effective for pulp capping. The composition with 400 ng TGF-beta-1 was able to trigger the resident stem cells to differentiate into odontoblast-like cells and induce the formation of tertiary dentin, suggesting that this material might be a good candidate for vital pulp therapy.
The application of anti-inflammatory factor(s) to caries-exposed pulp limits the inflammation, accelerates tissue regeneration, and leads to the deposition of mineralized dentin. The advantage of this approach is that the increased risk of pulpal necrosis or excessive calcification resulting from calcium hydroxide-induced tissue irritation is avoided.
CONCLUSION
the ability of the dental pulp to generate indirect and direct dentin and to regenerate should be kept in mind by clinicians. Indirect pulp capping or treatment (IPT) may be used in presence of deep carious lesion without pulp exposure (orthodentin or osteodentin). Beneath a calciotraumatic line, a layer of secondary tubular or atubular dentin is formed (DPC). Direct pulp capping may be used after pulp exposure, and a plug of osteodentin occludes pulp perforation (pulp bridge with or without tunnels, and/or calcospherites inside the pulp, diffuse pulp mineralization and/or pulp stones). Indirect or direct capping associate in therapeutic strategy three key elements, the same that are involved in tissue engineering. The association of (1) stem cells, (2) morphogens or growth factors, and (3) a scaffold of biomimetic extracellular matrix, might help the direct differentiation of dental stem cells and the subsequent regeneration of a functional dentin-pulp complex [32-33]. Apexogenesis and/or apexification are the two targets of pulp capping therapies, involving pulp regeneration.
No Files Found
Share Your Publication :