-
Publish Your Research/Review Articles in our High Quality Journal for just USD $99*+Taxes( *T&C Apply)
Offer Ends On
Michel Goldberg*
Corresponding Author: Michel Goldberg, Department of Oral Biology, Faculty of Fundamental and Biomedical Sciences, INSERM UMR-S1124, Paris Cité University, France.
Received: March 01, 2023 ; Revised: March 03, 2023 ; Accepted: March 06, 2023 ; Available Online: March 16, 2023
Citation: Goldberg M. (2023) Pulp Regeneration. J Oral Health Dent Res, 3(1): 1-10.
Copyrights: ©2023 Goldberg M. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Views & Citations
Likes & Shares
Pulp therapies imply indirect or direct pulp capping, vital pulp amputation, and treatment of non-vital teeth. Pulp necrosis therapy implicated pulp regeneration, in the crown and root(s). Dental stem cells isolated from permanent teeth and SHED from exfoliated deciduous teeth was used in order to regenerate the dental pulp. In the apical part of the root, the apical papilla stem cells (SCAP) include the cell-rich zone, and the adjacent apical papilla mesenchyme. The radicular dental pulp contributes to form either the reactionary parietal dentin or the reparative dentin. Stem cells moved laterally from the Höehl sub-odontoblastic border the odontoblasts located externally beneath the pre-dentin and the dentin. Afterwards, the stem cells are sliding of the root toward the crown. Following pulp penetration by cariogenic bacteria and the disinfection of the necrotic pulp the procedure consists in the mechanical enlargement of the root canal. Filling the pulp chamber by a continuous layer of pulp cells, linked by desmosome-like junctions, gap- and tight- junctions produce a hermetic seal of the cavity. A leak-tight filling associated with a biodegradable scaffold supports a permanent biological material closing the gap between the pulp chamber and secondary dentin. Cell-free scaffolds deliver bioactive molecules inducing cell differentiation. Pulp regeneration implicates tissue bioengineered. In the next future, this procedure may constitute a valid substitute to endodontic treatment.
Keywords: Pulp capping, Pulp regeneration, Stem cells, Höehl’s sub-odontoblasti layer, Intercellular junctions, Desmosome-like, Gap junctions, Tight junctions, Cell sliding, Leak tight filling
INTRODUCTION
Four different possibilities of endodontic therapies are used for dental pulp treatment. They include 1) indirect pulp capping, 2) direct pulp capping, 3) vital pulp amputation (pulpotomy), and 4) treatment of non-vital teeth (pulpectpmy) [1]. Taking origin from bone marrow mesenchyme, stem cells (BMMSCs) give rise to osteogenic, chondrogenic, and adipogenic SC. In addition, myogenic, neurogenic and tenogenic cells are derived from BMMSCs. Pulp repair is no more an unsolved problem. Stem cells have the capacity to self-renew and give rise to differentiated progeny. Regeneration using stem cells restore or re-establish a dental pulp that is no more alive. Indeed, a number of STEM cells penetrate the lumen of the canal, sliding from the peri-apex toward the crown.
Multiple signaling molecules, including BMPs, FGFs, Shh, and Wnt proteins, are implicated in mediating tissue interactions. Markers that are consistently reported are STRO-1, CD166 and CD 271, Oct4, Nanog, beta2 integrin, whereas CD14, CD34, CD45, and HLA-DR display negative expression. Regenerative medicine aims to repair and restore pulp structure and function. It requires re-vascularization and re-innervation, and new dentin deposition. Seeded on a poly-D,L-lactide/glycol scaffold, and transplanted into root canal space, stem cells are able to refill the pulp space with a newly formed pulpal tissue. A continuous layer of mineralized tissue of tertiary dentin is deposited along the pre-existing dental walls of the canals.
Stem cells have the capacity to self-renew as well as to give rise to differentiated progeny. They are responsible for normal tissue renewal and for regeneration following damage. Tissue engineering is considered as one of the most powerful approaches to repair an injured tissue or replace the pulp in the next future.
Dental pulp stem cells (DPSC) are obtained from deciduous exfoliated tooth, from permanent teeth (SHED), from the apical papilla (SCAP), from cementum-like cells, from the periodontal ligament (PDL), and SC of the follicular sac. SC may differentiate into odontoblasts, dental follicle, periodontal ligament, osteoblasts, adipocytes, and neurons.
Despite no more pulp canal cells are still alive, the few that survive may dedifferentiate and contribute to pulp regeneration. Thickening of the dentin layer occurs along the root walls, together with the apical SC which contribute to the closure of immature tooth. Apexogenesis provide lengthening of the root, and better anchorage inside the bonny socket (alveolar bone). Apexification induces the closure of the apex, and contributes to heal and regenerate the dental pulp.
Indirect pulp capping, selected extracellular matrix molecules promote the healing of the pulp. Direct pulp capping with Ca(OH)2, MTA, BMPs, Dentonin or other biological molecules [2] allows the formation of a reactionary/reparative dentinal bridge. In contrast, in presence containing degraded matrix and dead cells, pulp regeneration is a challenge for the future, actually expected, but still not a daily reality (Figure 1).
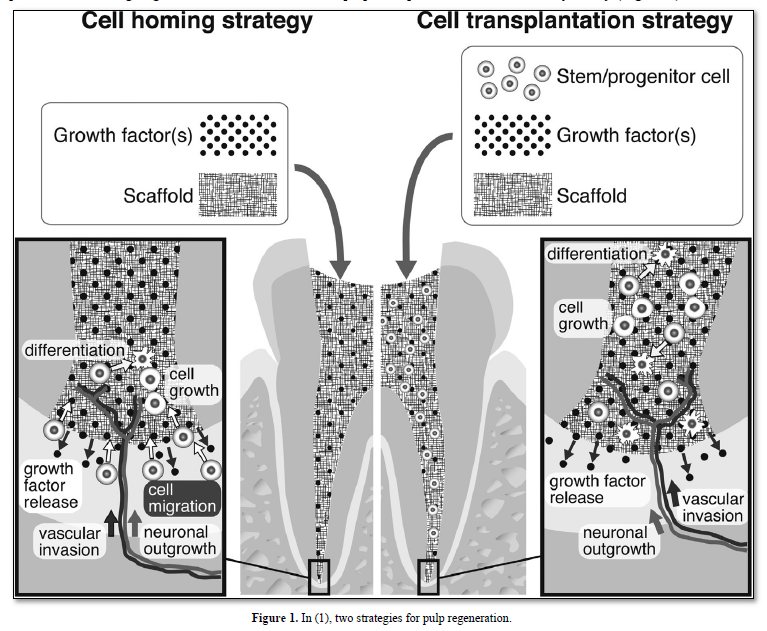
Odontoblasts may be partially or totally altered, depending on the virulence (pathogenicity) of carious bacteria and the diffusion of toxins along the dentin canaliculi. Scaffolds, growth factors such as the Basic Fibroblast Growth Factor, Transforming Growth Factor -TGF, Nerve Growth Factor, Platelet-Derived Growth Factor, Bone Morphogenetic Proteins and Stem Cells constitute efficient tools for regeneration. Odontogenic differentiation of hDPSCs and periodontal ligament cells may be promoted when cultured in a de-cellularized extracellular matrix scaffold. Characterization of the ECM scaffold showed that it contains a rich source of matrix proteins, metalloproteases and growth factors, having the capacity to regenerate the radicular pulp.
Up to now, the only efficient therapies were the partial pulpotomy or the total pulpectomy. The removal of the necrotic pulp and disinfection followed by an endodontic treatment and filling that leak tightly the pulp space of the root canal. Reparative pulp implicates physiological regeneration of the pulp tissue.
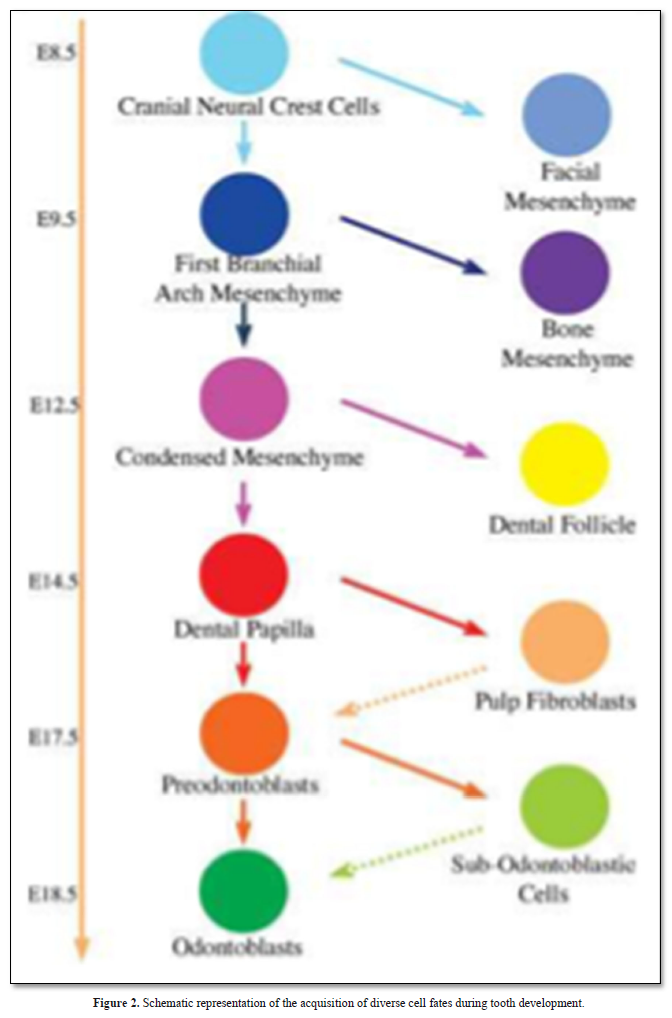
Regenerative therapy is a promising tool and a potential substitute to endodontic treatment. Observation of the molecular similarities that exist between tooth development and regeneration leads to dental pulp engineering and consequently to regenerative medicine. It can be expected that within the next two decades, the field of dentistry may be changed significantly by the availability of innovative tissue engineered products in dental office (Figure 2).
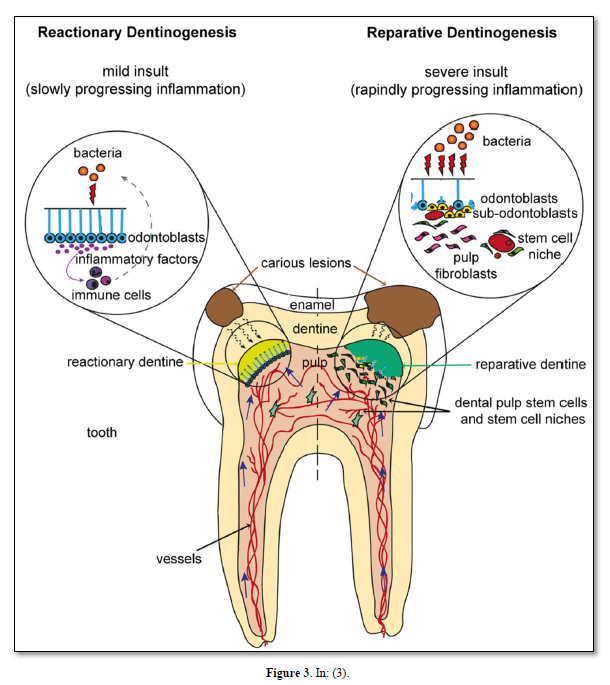
Physical interactions and molecular cross talk with stem cells, as well as the orientation of the cleavage plane during their mitosis, determine the fate of these cells. Molecules of the Wnt, Notch, and BMP pathways have been shown to control the balance between symmetric and asymmetric division of stem cells and related cell fate specification outcomes. This extracellular matrix is called reparative dentin (Figure 3).
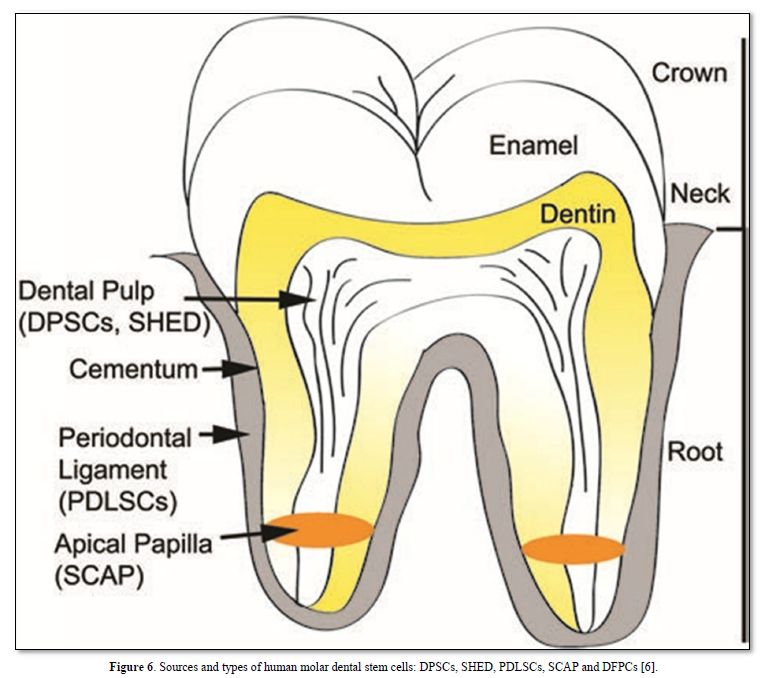
Sources and types of human dental stem cells
Bone-marrow-derived mesenchymal stem cells (BMMSCs) commit osteogenic differentiation in the dental pulp, dental stem cells (DPSCs) were isolated from permanent teeth and SHED from deciduous teeth. The apical papilla (SCAP) includes the apical cell-rich zone, the adjacent apical papilla mesenchyme and the radicular dental pulp. The periodontal ligament and cementum include stem cells having also regenerative potential.
The phenomenon and the process of ageing are now-a-day better understood. A conceptual framework has been established with general principles of ageing and longevity. The non-cell-based revitalization procedure leads to pulp/dentin regeneration. On the other hand, the cell-based approach regenerates pulp/dentin. Such approach will face barriers (4). Improvements were provided by (1) critical size defect of dentin and pulp, (2) cell lineage commitments to odontoblasts, (3) pulp repair, and (4) hurdles of cell-based pulp regeneration for clinical applications. Dental pulp stem cells from apical papilla and pulp stem cells from human exfoliated deciduous teeth demonstrate the capability to ‘de novo’ regenerate the pulp.
Pulp biology: pulp anatomy and cells characterization
General organization
Odontoblasts form the outer pulp layer. They are post-mitotic cells, together with Höehl’s cells characterized as differentiating progenitors. The dental pulp itself is located more centrally. Odontoblasts are the most important cells of the tooth, implicated in extracellular matrix synthesis and the gradual thickening of dentin layer. Primary and secondary dentins constitute the bulk of dentin. In reaction to the carious lesion, or as a repair process, the tertiary dentin (osteodentin) is formed.
Odontoblasts contribute to synthetize alpha-chains procollagen, associated and secreted as fibers in the extracellular and pericellular areas. The functions of Höehl’cells need still to be clarified. However, following the reduction of layers of odontoblast cell bodies, initially forming four to five rows. Growing older, and becoming mature, the odontoblast layers are reduced to a single one. They are migrating from Höehl’s cells [3-5]. Lateral migration of Höehl’s cells has been identified, both in the crown and in the root(s).
As an alternative possibility apical pro-genitors are stained by the TUNEL method and by the anti-transglutaminase antibody. Three different areas may be distinguished by PCNA (proliferating cells nuclear antigen) staining. The apical part of the root includes 1) the apical cell-rich zone, 2) the apical papilla mesenchyme, and 3) the radicular dental pulp. Cells slide from compartment 1 to 3.
The main processes of odontoblasts display bundles of cytoskeletal proteins such as microtubules, intermediary filaments, identified as vimentin and nestin, and actin microfilaments. Altogether, they contribute to a sub-plasmalemal undercoat. Odontoblast processes penetrate inside dentin tubules in the inner first 150 m, or penetrate the whole dentin thickness, in some cases, up to the dentino-enamel junction. The diameter of the lateral branching of odontoblast is smaller. The lateral branches do not contain nestin, but only vimentin and actin [7-10].
The sub-odontoblastic cells [11] have the ability to differentiate into hard tissue-forming cells, and may serve as a source for the renewal of odontoblastic cells (Figures 4 & 5).

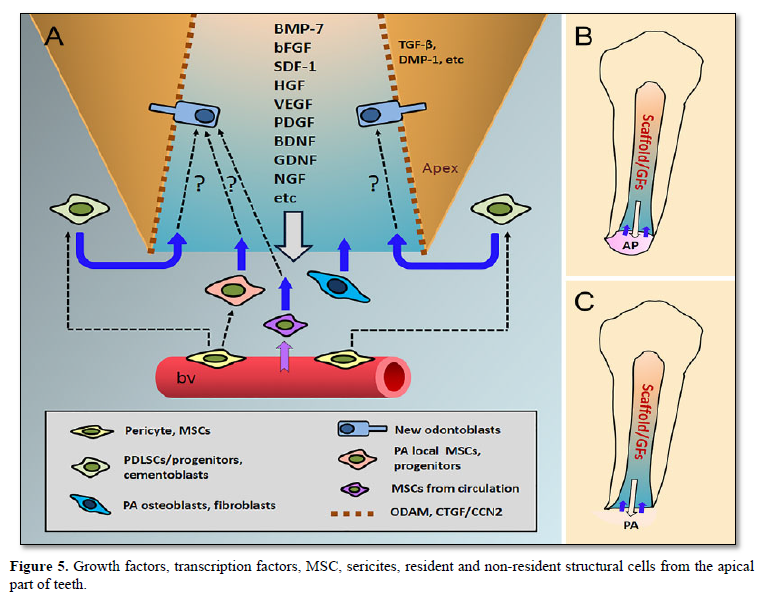
Resident and non-resident structural cells penetrate within the dental pulp through the apical foramen. Odontoblasts are subjected to renewal and apoptosis. According to Kenmotsu [12] between 0.11% to 0.40% of the pulp are stem cells A few non-resident cells take origin in the bone marrow or from the blood. They penetrate within the dental pulp through the apical foramen.
Each cell body may be divided into three: the basal third, the central third is the place where the nucleus is located. This part is implicated in immature vesicles formation. The distal (apical) third contains secretory vesicles.
Cell processes penetrate inside canaliculi, and the first 150m are implicated in extracellular matrix protein synthesis and secretion. This is also the part where mineralization occurs.
Growth factors
The DPSCs [13] are regulated by TGF, b- FGF, PDGF, EGF, TNF and IGF I and II.
bFGF is a modulator of cell proliferation. Located in chromosome 4. FGFR1 and FGFR2 are expressed in human dental pulp cells. TGF is divided into 3 isoforms (TGF1, 2, and 3). Nerve Growth Factor (NGF) regulator in the development, survival, differentiation and maintenance of neuronal and non-neuronal cells. NGF was found to be expressed in dental tissue, undergoing cell proliferation and odontogenesis. Platelet-derived GF identified in the cell-free plasma. The gene encoding the PDGF-A chain is located on chromosome 7, while the PDGF -B is located on chromosome 22. The PDGF-C and PDGF-D genes are located on chromosomes 4 and 11. PDG-F enhances DPSC proliferation, odontoblast differentiation, and the regeneration of dentin–pulp complex. Bone Morphogenic Proteins (BMPs) induce bone formation. BMP2-transfected DPSCs isolated by STRO-1 revealed high levels of alkaline phosphatase (ALP) activity in vitro and the enhancement of mineralized tissue upon implantation. BMP4 affects the growth of dental pulp cells and enhances the mRNA expression levels of ALP, DSPP, and DMP-1. BMP7 induction resulted in increases in dentin sialo phosphoprotein (DSPP), osteocalcin (OCN), dentin matrix protein 1 (DMP- 1), and runt-related transcription factor 2 (RUNX2) mRNA expression levels and the formation of mineralized nodules in DPSCs (Figure 6).
Human dental pulp stem cells (SCAP) and DPSCs were isolated, seeded onto synthetic scaffolds and transplanted into the empty root canal space of a mouse molar. The transplantation resulted in the formation of vascularized pulp-like tissue and the deposition of a layer of dentin-like tissue. Although the dentin layer was not well organized and no long-term functionality of the regenerated tissues was tested. This study provided the first example of de novo dental pulp regeneration.
Markers used for identifying MSCs are also used for dental stem cells. Positive markers: such as CD146, Oct4, Nanog and β2 integrin, and negative markers: CD14, CD34, CD45 and HLA-DR [7-10].
Pulp fibroblasts, also named pulpoblasts by Baume [9]. This suggests that there is gradual elimination of cells, together with secondary dentin deposition.
This provides some evidence that odontoblasts division and sliding occurs, younger cells playing role in the replacement of older cells.
Intracellular proteins
Positive immunostaining was seen for vimentin and 1 type 1 collagen. In contrast, the staining for CD45, CD34 and cytokeratin was negative as evidenced by flow cytometry. The cytoskeleton was well developed. Connexin 43 is a 43-kDa gap-junction protein forming hexametric structures creating intercellular channels and allowing cell-to-cell diffusion of small molecules. Pulp cells transmit information as a syncytium via gap junctions. Lucifer yellow stains the superficial pulp cells [16]. Specific immunolabeling revealed smooth muscle actin and intermediary filaments, namely vimentin, gap-junctions [17]. TGF enhances the expression of smooth muscle actin in cultured human pulpal fibroblasts [18]. Nestin is also an intermediary filament. It was reported to be specific of the odontoblast lineage. This seems to be true for some species, but erroneous for some other groups of animals where vascular endothelial cells, nerves and other type of cells reacted also positively to nestin antibodies.
Smad 1/5, Noggin, histone desacetylase inhibitors, pluripotin (SC1), bio and rapamycin are intracellular proteins.
Non-resident pulp cells have the potential to differentiate into three skeletal phenotypes MSCs are lacking expression of the hematopoietic markers CD45, CD14, CD34, HLA-DR, and CD31, an endothelial marker. They are expressing CD105, CD73, and CD90. ECM is composed by thin collagen fibrils (type I -56%, III (41%) and V (2%) (CS-4 and 6 DS and KS), DSP, glycosaminoglycans associated with a link protein (proteoglycan), biglycan, decorin, versican, MMP-2, MMP-9, TIMP-2. Period-2 (PER 2), Period-3, osteoadherin, glypican-3, midkine, activine receptor interacting protein-1 (AIP 1), growth hormone receptor (GRH), adrenomedullin (ADM), interleukin-11, BSP, matrix Gla protein (MGP), endothelial cell growth factor-1 (ECGF 1), inhibin beta A and oromucoid-1 were identified in diseased pulp [16-27].
Immune defense mechanisms of the dental pulp: B-lymphocytes and T-lymphocytes subdivided into T-helper cells (CD4+) and cytotoxic T-Cells (CD8+). These cells are natural killers (NK), producing chemokines and inflammatory cytokines such as interferon and tumor necrosis factor.
Pulpal dendritic cells (DCs), provide the necessary signals, which activate T-lymphocytes. DCs have elongated cytoplasmic processes and contain a few lysosomal structures.
Macrophages form a group of cells including a series of bone marrow-derived cells comprising monoblasts, promonocytes, monocytes in the peripheral blood, and diverse populations of recognized functionally as macrophages, Mast cells. polymorphonuclear leukocytes (PMNs) containing azurophilic granules of PMN elastase, cathepsin-G and alpha-2 macroglobulin (alpha-2M).
Microvasculature
As seen in corrosion resin casts of dog pulp, in the crown, the sub-odontoblastic layer are fed by capillaries forming successive glomerular well-individualized structures that feed limited areas about 100-150 m wide. This implies that a pulp lesion provokes controlled diffusion in the crown. Bypass or arteriovenous anastomoses favor in the crown the healing of a restricted inflammatory pulp area. After a lesion or in case of infection, the radicular pulp underwent necrosis, irreversibly. The inflammatory process diffuses and invades the whole radicular pulp, following a fish net pathway.
The lymphatic system plays a crucial role in the immune barrier function and for tissue fluid balance. The transport of stimulate lymphangiogenesis. Lymphatic capillaries were identified under local anesthesia after injection in the pulp horn of 0.2 to 0.3 cc of sterile colloidal carbon. Capillaries lymphatics are characterized by occasional large intercellular clefts, absence or incompleteness of basement membrane, absence of pericytes, filamentous material between the endothelium and the surrounding collagen fibrils [30]. Using anti-Prox-1 an antibody specific for lymphatic vessels, they were unable to detect any vascular structure lined by lymphatic endothelium. Berggreen [32] found positive immunostaining using antibodies rose against the lymphatic endothelial hyaluronan receptor-1 and vascular endothelial growth factor receptor-3 in the coronal molar pulp, whereas in the incisor immunostaining was found only in the apical part. These observations suggest that macrophages contribute directly to the formation of lymphatic vessels after pulp exposure. A subfraction of cells from pulp is a source for cell-based therapy stimulating angiogenesis/ vasculogenesis during tissue regeneration [33].
Innervation
Nerves penetrate through the apex. They fan out in the crown forming the sub-odontoblastic Raschkow’s plexus [34]. Nerve terminal endings establish close contact with the plasma membrane of the odontoblasts and sub-odontoblastic layer. Junctions of the gap type provide continuity between nerves ending containing synaptic vesicles (small electron lucent and electron dense vesicles, and/or large vesicles), and the plasma membrane of the odontoblasts. Some axons penetrate the predentin between odontoblast. They cross the predentin, and enter in the inner third of dentin tubules, associated in the lumen with odontoblast processes.
Myelinated A-Fibres
Unmyelinated C-Fibres
CONCLUSIONS
Under appropriate conditions, a pool of stem cells inside the pulp can be activated. DPSC and SHED are produced (permanent and deciduous. SCAP contribute to maintain apical progenitors. These cells slide from the apex toward the crown. Along to the dentin, odontoblasts slide from the Höehl layer(s) to the sub-odontoblastic rows, and beneath the calcio-traumatic line, contributing to the formation of secondary dentin. Regeneration of a thin tertiary dentin layer is followed by disinfection, pulp regeneration and narrowing of the pulp volume. The newly formed dental pulp is reduced gradually in size (width and thickness). Apical closure, associated to pulp regeneration, contribute to pulp repair. This allows tissue engineering, a potential substitute to endodontic treatment. Stem cells have the capability to regenerate into a functional dental tissues, or they may restore the function of diseased/injured tissues. Regeneration of human tooth may be a realistic approach [36]. There is still the open question of the ideal scaffolds and the induction of adequate neovascularization. Further researches in this area are needed in order to surpass all these obstacles, as well as cooperation with other disciplines. The introduction of dental stem cell-based therapy in clinical work would revolutionize regenerative dentistry [37].
No Files Found
Share Your Publication :