-
Publish Your Research/Review Articles in our High Quality Journal for just USD $99*+Taxes( *T&C Apply)
Offer Ends On
Michel Goldberg*
Corresponding Author: Michel Goldberg, Department of Oral Biology, Faculty of Fundamental and Biomedical Sciences, INSERM UMR-S1124, Paris Cité University, France.
Received: January 12, 2023 ; Revised: January 27, 2023 ; Accepted: January 30, 2023 ; Available Online: February 16, 2023
Citation: Goldberg M. (2023) Mechanisms of Tooth Eruption. J Oral Health Dent Res, 3(1): 1-8.
Copyrights: ©2023 Goldberg M. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Views & Citations
Likes & Shares
Tooth eruption is a complex multifactorial process. Association of alveolar bone remodeling, root elongation, cementum apposition, and periodontal ligament formation are associated to tooth eruption. After an initial pre-eruptive phase, pre-occlusal and post-occlusal eruption is occurring. Then, the tooth becomes functional. Gubernacular cord and canal are involved in the tooth eruption process. This includes transcription and growth factors and hormones such as IL-1 and PTHrP. They are both located in the stratum reticulum (SR). The presence of metalloproteinases suggests that tooth eruption is also implicated in these processes. Genesis of osteoclasts and osteoblasts is related to these events. Up to now, a single process has not been identified, but complex effects combine together and shed lights on different causes of eruption. Apexification (root lengthening) and apexogenesis (apical closure) are two distinct causes and effects of tooth eruption.
Keywords: Alveolar bone, Pre-eruptive tooth, Eruption, Periodontal ligament, Cementum apposition, Stellated stratum, Gubernaculum, Transcription and growth factors, Osteoblasts, Osteoclasts, Metalloproteinases, Apexification, Apexognesis
INTRODUCTION
Tooth eruption is a continuous multifactorial process, associated with alveolar bone remodeling, root elongation, cementum apposition and periodontal ligament formation.
There is no consensus on the mechanisms involved, however they include five successive stages:
Other events are also implicated: such as pulpal pressure and growth, root lengthening, traction by periodontal fibroblasts, acellular and cellular cementum formation, and vascular pressure.
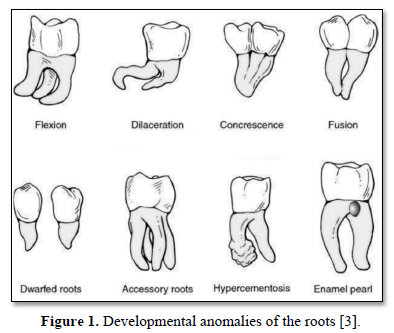
Developmental anomalies of tooth are recognized, including flexion, dilacerations, concrescence, fusion, dwarted roots, accessory roots, hypercementosis, and enamel pearl (Figure 1).
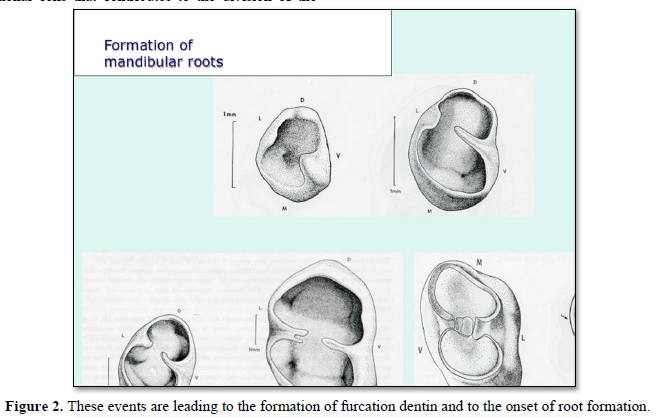
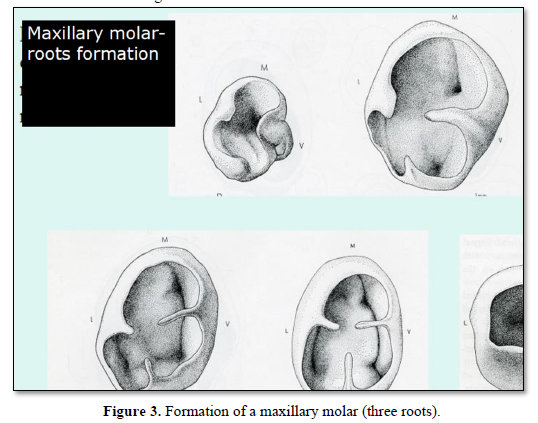
After the formation of the crown, from the cervical region (labial or lingual parts) starts the proliferation of a tongue of epithelial cells that contributes to the division of the cervical space into two (for mandibular molars) or three divisions of the cervical space [4] (Figures 2 & 3).
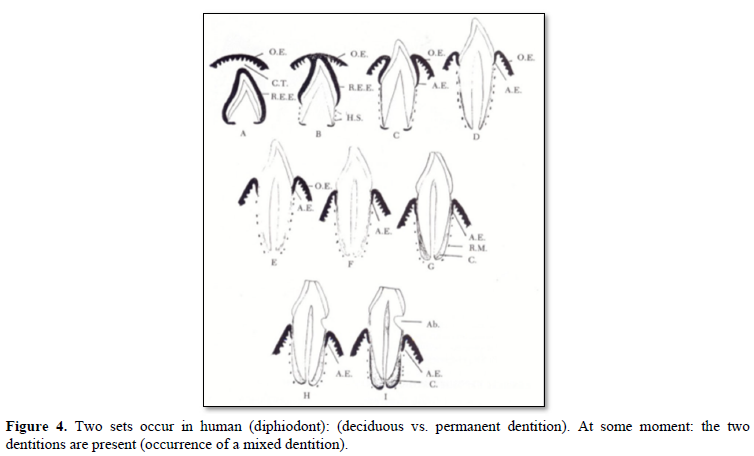
Tooth eruption implies 3 different phases (Figure 4):
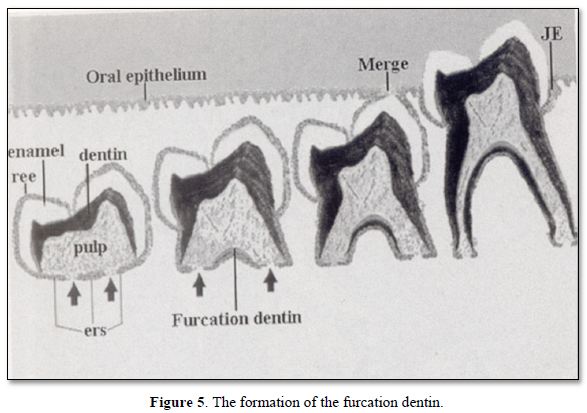
The formation of the furcation dentin, followed by the merge and the occurrence of contacts between lower and upper molars. This implies the end of the tooth eruption (Figure 5).
Four erupting tooth movements are recognized. They are due to:
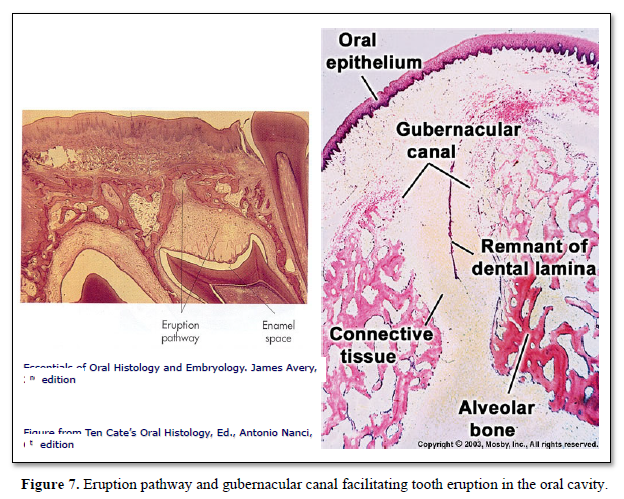
Gubernacular cord and canal are involved in tooth eruption: Widening of the gubernacular canal allows the eruption of the tooth. The rate of tooth eruption is related to two different phases:
Mechanisms of eruptive movement involve a multifactorial process due to:
Active eruption and Passive eruption: Recession of the gingiva and the underlying alveolar bone four events are implicated in tooth eruption.
2. Bone remodeling.
3. Defects in osteoclast differentiation.
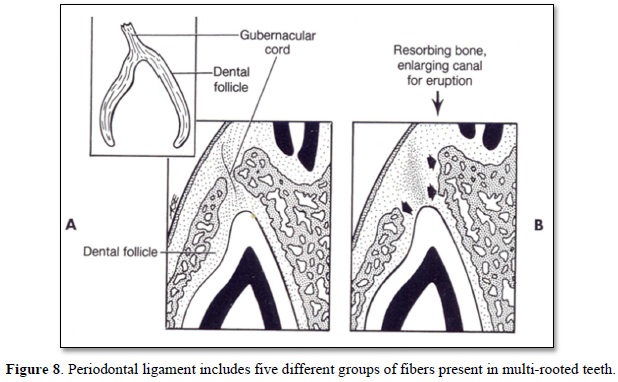
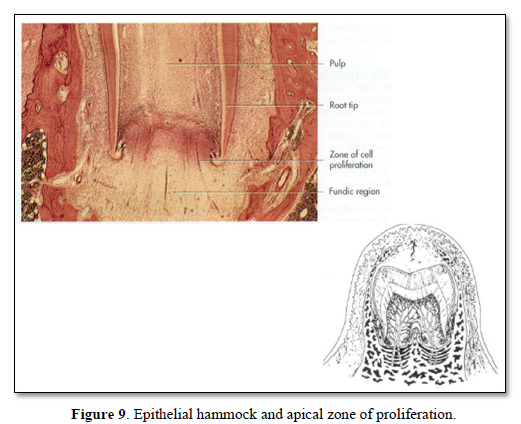
4. Periodontal ligament (PDL) is present, but the tooth is not erupting, however, it is obvious that rootless teeth are erupting, despite the lack of root formation (Figures 8,9).
5. Role of dental follicle: Cellular events - Molecules involved in tooth eruption:
Dental Follicle-95 is selectively degraded at the onset of tooth eruption [5]. Eruption molecules are implicated in tooth movements: EGF, TGF- and EGF utilize the same receptor. The Colony-stimulating factor-1 (CSF-1) is also contributing to tooth migration. The presence of IL-1 and PTHrP in the stratum reticulum (SR) suggests that the SR portion of the enamel organ plays a role in eruption; DF-95 is present at the onset of eruption and then decline. Analyses at different stages of premolar eruption indicate that selective fragmentation of dental follicle protein DF-95 correlates with the presence of elevated levels of follicular collagenase and stromelysin [6].
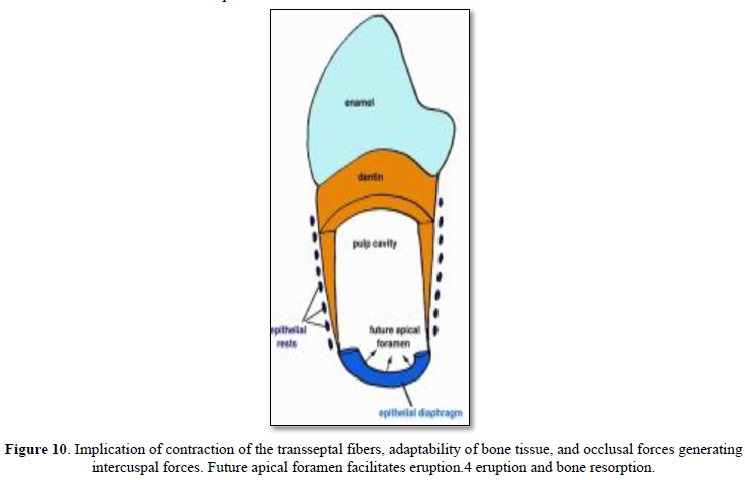
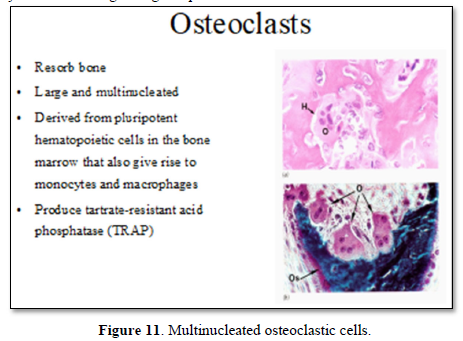
Mononuclear cells (osteoclast precursors) must be recruited into the dental follicle prior to the onset of eruption. These cells fuse to form osteoclasts that resorb alveolar bone, forming an eruption pathway for the tooth to exit its bony crypt (Figure 10).
Recruitment of the mononuclear cells to the follicle may require colony-stimulating factor-one (CSF-1) and/or monocyte chemotactic protein-1 (MCP-1). Osteoclastogenesis is needed for the bone resorption and enhancement of receptor activator of NFκB ligand (RANKL), in the adjacent alveolar bone and/or in the follicle. Paracrine signaling by parathyroid-hormone-related protein and interleukin-1α, produced in the stellate reticulu may also play a role in regulating eruption. Osteoblasts might also influence the process of eruption, the most important physiologic role likely being at the eruptive site, in the formation of osteoclasts through signaling via the RANKL/OPG pathway. Evidence supports a role for an osteoblast-specific transcription factor, Cbfa1 (Runx2), in molecular events that regulate tooth eruption. Cbfa1 is also expressed at high levels by the dental follicle cells [7].
Putative Tooth Eruption Molecules
EGF, EGF-R CSF-1, CSF-R, IL -1, IL-1R, c-Fos, NFkB
MCP-1, TGF-1, PTHrP, Cbfa1, OPG/OCIF, RANKL
Fibroblasts may play a possible role in tooth eruption. Interstitial fluid pressure may also be related to tooth eruption.
Mononuclear Osteoblasts differentiation Osf2 or Cbfa1 (Core-binding factor a1) is a key transcriptional regulator of osteoblast differentiation during bone formation.
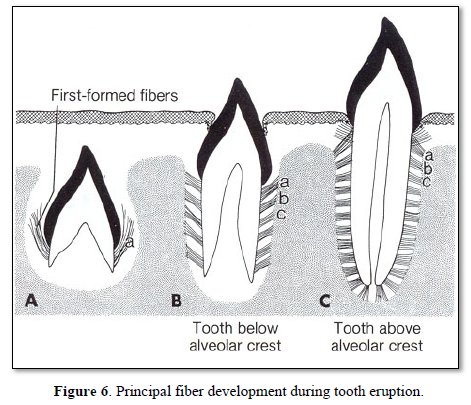
In single rooted teeth, groups of fibers are forming:
Lately, molars are sliding mesially.
Control factors of the mesial drift:
Eruption and bone resorption
Intrapulpal blood pressure
Maturation / Migration fibroblasts, contraction of collagen gels; Maturation from the native collagen to mature collagen fibers (1000 A of the native collagen, shortening to 670 m in length of the mature collagen, after enzymatic ablation of the N- and C- termini).
6. Changes in alveolar bone: First, compression sites are organized.
Second, resorption occurs at compression sites. Many mechanisms have been reported, such as root elongation, or/and bone deposition. None of these factors seems to be a sufficient cause for tooth eruption [7].
7. Bone growth: Marks and Cahill [8] interpreted their experiments, with the eruption of metal and silicone replica, providing evidence for alveolar bone growth (apposition and resorption) passively 'carrying' the tooth through bone into function.
8. Periodontal ligament
From a biomaterial perspective, the periodontal ligament is a complex, fiber-reinforced substance that responds to force in a viscoelastic and non-linear manner.
9. Adjacent alveolar bone: A bone remodeling cycle has a number of phases: activation, resorption, reversal, and formation. Inhibition of the molecules that promote osteoclastogenesis would serve to prevent and inhibit eruption.
Numerous reports indicated as key osteoclastogenic molecules, such as RANKL, osteoprotegerin, and VEGF are expressed in the periodontal ligament. The osteoprotegerin is secreted in vitro fibroblasts can inhibit osteoclast formation. During a period of tooth eruption, only the expression of osteoprotegerin need to be inhibited and bone resorption is occurring.
The first evidence of eruption is bone resorption beneath the calcified crown, in a direction opposite to the one in which the tooth is expected to move. Such cellular activity is not unexpected if a significant 'origin' of force within the follicle becomes displaced apically subsequent to calcification of the crown. The mechanics of tooth eruption lead to the conclusion that forces from the apical vasculature are the likely source of the eruptive force. As tooth eruption can be explained by the action of forces acting in a dynamic relationship with bone remodeling, many factors modify the rate and direction of the process.
All these modifications are influenced by genes that expressed molecules such as:
10. Hormones and growth factors: Growth Factor receptors and parathyroïd related protein (PTHrP). Indeed, the parathyroid hormone–related protein (PTHrP) and its parathyroid hormone/PTHrP receptor PPR signaling appear to crosstalk with other signaling pathways.
11. Enzymes: (Protein-kinase C; PKA) May play a role in tooth eruption.
12. MetalloProteinases: MMP-8 and MMP-13 and Disintegrins (PGs are cleaved: e.g. versican is cleaved by ADAMTS 1, ADAMTS 4, ADAMTS 5) [9].
13. Potential role of MT1-MMP: The MT1-MMP activity in the dental mesenchyme is essential for proper tooth root formation and eruption. These studies point to an indispensable role for MT1-MMP-mediated matrix remodeling in tooth eruption through effects on bone formation, soft tissue remodeling and organization of the follicle/PDL region.
MT1-MMP, a transmembrane zinc-endopeptidase that breaks down extracellular matrix components, indicates that MT1-MMP activity in the dental mesenchyme is essential for proper tooth root formation and eruption. These studies point to a role for MT1-MMP-mediated matrix remodeling in tooth eruption through effects on bone formation, soft tissue remodeling and organization of the follicle/PDL region [10]. In addition, resorption of deciduous teeth (by dentinoclasts) is associated to the eruption of permanent teeth.
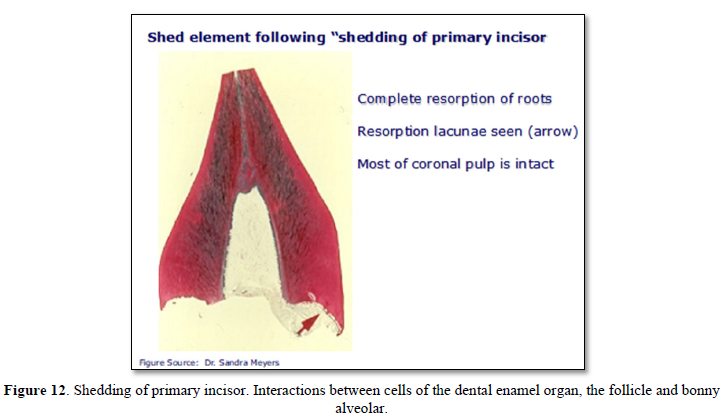
Failure of Eruption (PFE) (apical closure): Eruption results from interactions between cells of the dental enamel organ, and alveolar bone (Figures 11 & 12).
CONCLUSIONS
Tooth eruption is a multifactorial event. Collagen maturation constitutes the basis for periodontal ligament maturation. Ablation of the collagen C and N termini leads to a reduced length of fibers initially synthetized as pro-collagen (1000 A), then reduced to a 670 Å periodicity (mature collagen). This shrinkage may contribute to maturation and consequently to tooth eruption. Changes were also identified in hormones (PTHrP), growth and transcription factors (TNF -a, -b, CSF-1, RANKL) metalloproteinases (MMP-8, 13, disintegrins, MT1-MMPs), enzymes expression of bone, expressed inside the ligament and in cementum (acellular and cellular enzymes expression at the surface of the bonny socket). The expression of proteases seems also to be crucial (root lengthening). Membrane localization of Baz, Par6-a PKC and Crb depend on Baz. Cdc-42 drives morphogenesis by conferring apical identity of Par6 [11]. The apical STEM cells contribute to tooth eruption. The process is not limited to root lengthening, but involves also some other factors regulating root morphogenesis (namely apexification, or apical closure).
No Files Found
Share Your Publication :