-
Publish Your Research/Review Articles in our High Quality Journal for just USD $99*+Taxes( *T&C Apply)
Offer Ends On
Michel Goldberg*
Corresponding Author: Michel Goldberg, Department of Oral Biology, Faculty of Fundamental and Biomedical Sciences, INSERM UMR-S1124, Paris Cité University, France.
Received: June 05, 2023 ; Revised: June 08, 2023 ; Accepted: June 11, 2023 ; Available Online: June 16, 2023
Citation: Goldberg M. (2023) Osteoclasts: Dual Roles for Bone Cells Migration, and Degradation of the Extracellular Matrix. J Oral Health Dent Res, 3(3): 1-10.
Copyrights: ©2023 Goldberg M. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Views & Citations
Likes & Shares
Progenitors of bone cells (bone lining cells and pre-osteoblasts), osteoblasts and osteocytes are expressing chemokines, prostaglandins and growth factors (BMPs, TGF-CSF-1, G-CSF-, basic FGF, and IGF). The other group of bone cells is composed by osteoclasts (OC), derived from hematopoietic stem cells and monocyte/macrophage lineage cells. They play role in cellular senescence, appear as progenitor cells included in bone marrow. OC are also implicated in bone resorption and decomposition of the extracellular matrix. M-CSF (macrophage colony-stimulating factors), and IL-34 are ligands of colony stimulating factor 1 receptors, modulating macrophage activation. OC are multinucleated cells (between 10 and 20 nuclei). Interleukin vstimulates the differentiation of OC progenitors. The RANK signaling pathway contributes to OC activation. They migrate at the surface of bone and they are involved in a resorption phase creating osteoclastic lacuna. OC are limiting the sealing zone, surrounded by an actin-ring organizing podosomes in the clear zone. OC are loaded by small vesicles and mitochondria. Chloride/ bicarbonate provides insights in the mechanisms of osteoclastogenesis. Cathepsin K, MMP-9 and TRAP are enzymes implicated in the degradation of the extracellular matrix. Degraded matrix components are endocytosed. Transcytotic vesicles secrete acid and lytic enzymes. Howship’s lacuna includes secretory lysosomes merging with the plasma membrane, limiting a sealing zone implicated in bone resorption. They initiate the secretion of parathyroid hormone (PTH) and related peptide (PTHrP). OC are implicated in pathological conditions including osteoporosis, Paget’s disease, rheumatoid arthritis, and periodontitis.
Keywords: Osteoblasts, Osteocytes, Bone lining cells, Macrophage activation, Clear zone, Howship’s lacuna, Osteoclastogenesis, Cathepsin K, MMP-9, TRAP, Degradation of the extracellular matrix, Alveolar bone, Osteopososis, Paget’s disease, Periodontal disease, Aging
BONE CELLS AND INITIAL FORMATION OF THE MANDIBLE AND MAXILLARY
The initial formation of the mandible implies a series of two groups of cells
The mandible is formed initially by the Meckel’s cartilage. Afterward, a series of cartilages contribute to the formation of bone (condylar, coronoid, angular, mental), leading to the formation and mineralization of endochondral bone (Figure 1).
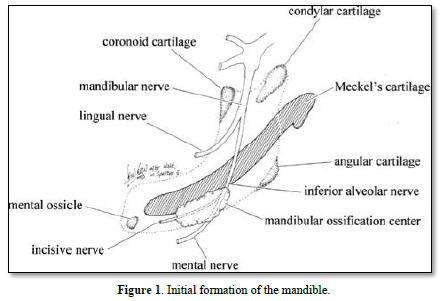
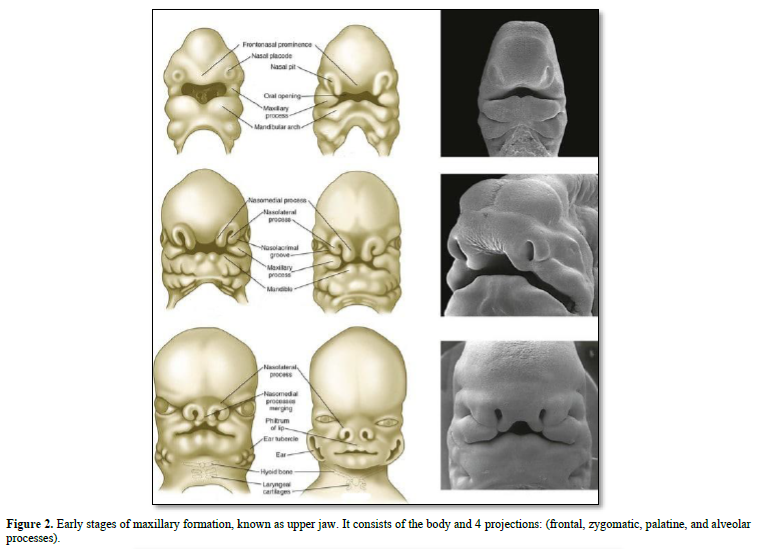
The maxillary bones are formed by the association of fronto-nasal process, the medial nasal, lateral nasal, all being located above the maxillary process. Fusion between the right and left parts of the maxillary occurs in the median nasal process. The maxillary bone is implicated in the formation and mineralization of membranous bone.
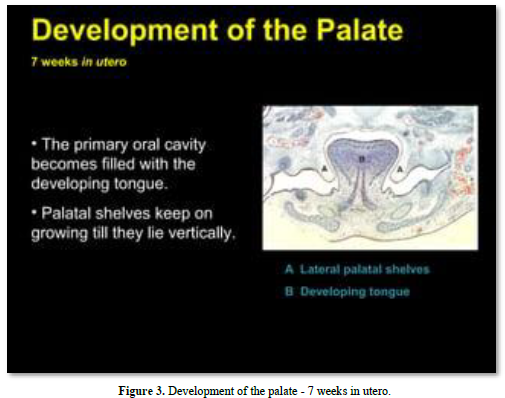
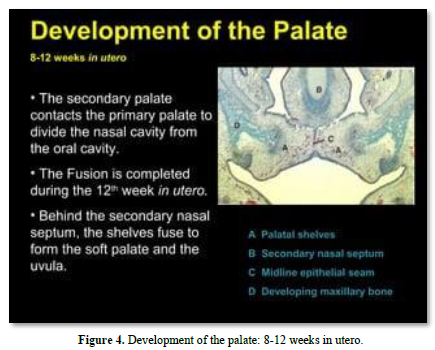
Meckel’s cartilage constitutes the initial structure of the mandible. A series of cartilages (coronoid, condylar; angular) are the initial places of endochondral mineralization. Membranous ossification is the other mode of mineralization, leading to mandibular formation and mineralization (Figures 2-4).
The different types of bone cells
Two types of bones are identified in the cranial skeletal system Cortical and trabecular bones.
In the mandibular bone, two main parts are present:
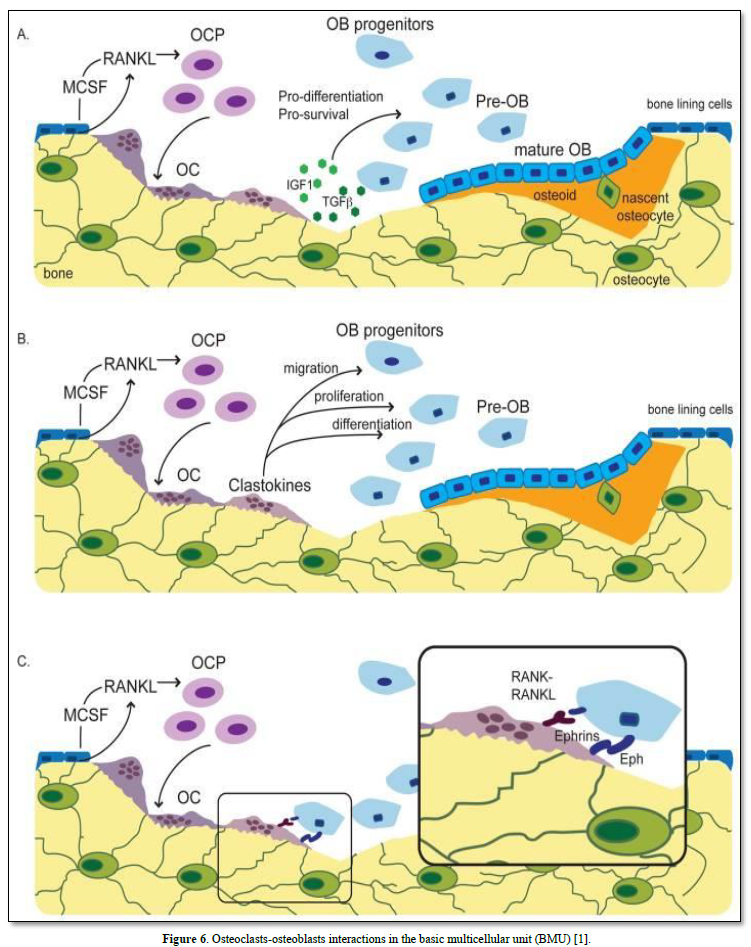
Figure 7. Osteoclasts: (20-100 μm): The outer membrane of stromal cells (αvß3integrin), bone lining cells. RANKL and calcitonin receptors are enzymes secreted: (cathepsin K, MMP-9, TRAP). Integrins (αvß3) and ruffle border are limiting a clear zone. The sealing zone forms an actin ring surrounding a ruffled border isolating the resorptive space from the surroundings.
Multinucleated cells formed by the fusion of mononuclear preodontoclasts. The cells migrate and differentiate from the spleen, or bone marrow, or differentiate from monocyte/macrophage lineage (M-CSF 5 (RANKL) (Figure 8).
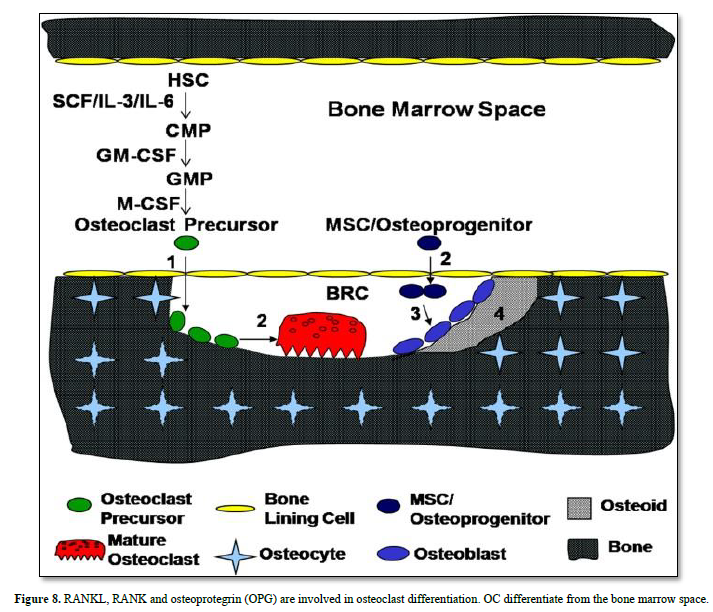
Bone lining cells expressing RANK are implicated in the differentiation of OC precursor, becoming mature OC. Osteoprogenitors (MSC) become osteoblasts, involved in osteoid and bone formation (containing osteocyte) (Figures 9 & 10).
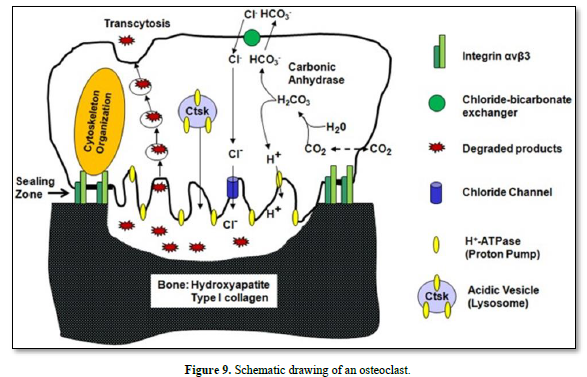
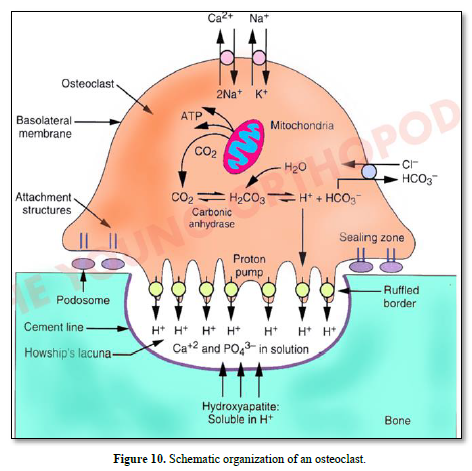
Vacuolar H+-adenosine triphosphatase (H+-ATPase present in the ruffle border membrane. This is accomplished by a passive chloride bicarbonate exchanger in the basolateral membrane.
Degraded products are endocytosed, and then transported along a transcytotic vesicular pathway toward the anti-resorptive side of the cell, and finally released out of the cell.
STRUCTURE AND FUNCTIONS OF OSTEOCLASTS
Osteoclasts are multinucleated cells (8-100 nuclei), that arise from hematopoietic stem cells (200 micrometers large). As a result, they are identifiable with the presence of CD13, CD14, and CD68 antigens and the lack of CD56, GrB, and Ki67 antigens. Activated through their receptor activator nuclear factor-kB (RANK), the large dome-shaped multinuclear cells are composed of fused mononuclear osteoclasts progenitor cells.
The inferior surface contains high concentration of vesicles. OC contain many mitochondria. In addition, Golgi bodies and lysosomes are also present in OC. The cells are subdivided in five: pre-osteoclasts (precursor or progenitors), active OC, Inactive OC, resting OC, and post-OC.
Osteoclatogenesis
The ruffled border has a high concentration of vesicles, which help acidify and secrete enzymes into the microenvironment formed by the sealed zone.
The lacunae in which osteoclasts are contained involve resorbing bone. They are known as Howship's lacuna. Lying under the ruffled membrane, they are loaded with numerous visible vacuoles. The vacuoles-containing cells are undergoing apoptosis.
Pathophysiology: The RANK-RANKL interaction is essential for osteoclastogenesis, and that interaction is under the regulation of osteoprotegerin (OPG). Osteoblasts and stromal cells secrete OPG, and it binds to RANKL on osteoblasts and stromal stem cells and prevents the interaction between RANKL and RANK, inhibiting osteoclastogenesis. This serves as a control mechanism to prevent excess bone resorption from taking place. OPG opposes the effects of 1, 25-dihydroxy-vitamin D, parathyroid hormone (PTH), parathyroid hormone-related peptide (PTH-rP), and prostaglandin E2 (PGE), which all increase bone resorption.
Therefore, the interplay between RANK-RANKL-OPG is crucial in regulating osteoclastogenesis and the maintenance of bone mineral density and plasma calcium levels. Dysregulation of the RANK-RANKL-OPG system plays a vital role in the pathogenesis of many conditions, such as osteoporosis, osteopetrosis, and bone tumors! [2].
The generated conditional knockout mouse model in which Stat3 was deleted in osteoclasts using a cathepsin K-Cre driver [3]. We observed that osteoclast-specific Stat3 deficiency caused increased bone mass in mice, which we attributed to impaired bone catabolism by osteoclasts. Stat3-deficient bone marrow macrophages showed decreased expression of nuclear factor of activated T cells, cytoplasm 1, and reduced osteoclast differentiation determined by decreases in osteoclast number, tartrate-resistant acid phosphatase activity, and expression of osteoclast marker genes [4].
BONE PATHOLOGIES LINKED TO OC ACTIVITIES
Osteoporosis
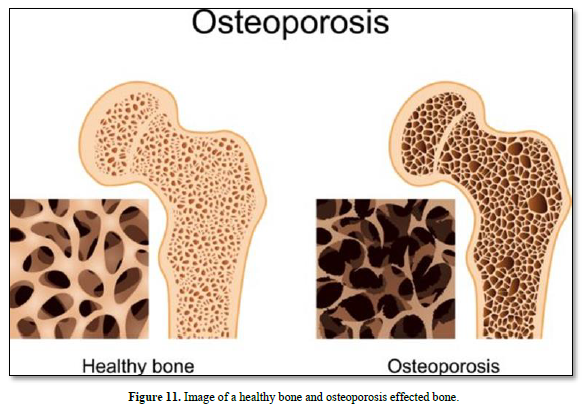
Approximately 10 million man in USA have osteoporosis, characterized by low bone density and deterioration of bone architecture that increase the risk of fracture. Bones become weak and brittle. Fractures occur in the hip, wrist or spine. Bone is broken down and replaced. Women are more likely to develop osteoporosis than men. The greater risk of osteoporosis is related to age. The diagnostic of osteoporosis is determined by measuring bone mineral density. Medications include estrogen agonist/antagonist, PTH hormone analogues, and calcitonin. Alendronate and risedronate are the most cost-effective in women.
Sex hormone level tend to weaken bone. Menopause is one of the strongest risk factors for developing osteoporosis. Parathyroîd reduces thyroid medication. Adrenal glands may be overactive. Low calcium intake, and gastrointestinal surgery; cortico-steroid medication (such as prednisone and cortisone). There is a need of 1.000 milligrams of calcium a day. The daily amount increases to 1200 milligrams when women turn 50 and men turn 70.
The disease increases in women after age 55 years and men after 65 years. Osteoporosis means “porous bone”. There is an increased non-modifiable risk of falls. However, bone may be potentially modifiable: alcohol and tobacco may prevent osteoporosis (Figure 11).
Paget osseous disease
Increase of volume (osseous hypertrophy)- Some bones are modified (alterations), while others appear intact.
Osteopetrosis
Also known as Albers-Schönberg disease (or marble bone disease, bone becoming denser and more brittle. Bone treatment with stem cell transplantation contribute to osteopetrosis transplantation.
The lack of carbonic anhydrase leads to inability to acidify Howship lacunae in bone.
There is a defect in. Infantile autosomal recessive osteopetrosis, and adult autosomal dominant are actors of osteopetrosis.
Gene implicated: the mutation of the gene CLCN7 is located on chromosome 16p13. Two main forms of osteopetrosis, are correlated with dysfunctions of OC: dysfunctions form is detected in young or adult patients. Alteration of growth is detected in the young form.
Osteogenesis imperfecta
Characterized by blue sclera, type I collagen, 1 in 15.000 -20.000 people, therefore it is a rare genetic disease.
Periodontal disease (or gum disease)
Developmentally there are two modes of bone formation: endochondral bone> compact (cortical) bone and Intramembranous bone spongy (cancellous) bone.
Causes are plaque forms on teeth, and afterward it turns into tartar.
Anatomy: Lamella concentric and interstitial lamella
The lacuno-canalicular network architecture connect osteocytes [5]. The morphology of the network allows connections between osteocytes.
Volkmann perforating canal
OC, the principal bone resorptive cell, differentiates from monocyte/macrophage precursors under the regulation of the critical cytokines/macrophage promote osteoclast activity, particularly in states of inflammatory osteolysis [6].
The pathogenic processes of destructive inflammatory periodontal diseases are instigated by subgingival plaque microflora and factors such as lipopolysaccharides derived from specific pathogens. These are propagated by host inflammatory and immune cell influences, and the activation of T and B cells initiates the adaptive immune response via regulation of the Th1-Th2-Th17 regulatory axis [7].
The dissolution of the mineral phase in the acidic microenvironment below the RB exposes collagen fibrils to the enzymatic attack of cathepsins B, E, K, S, and L. These cysteine proteases are secreted by osteoclasts to degrade native collagen at an acidic pH of 4.5. Thereafter, matrix metalloproteinases (MMPs), such as gelatinase A (MMP-2), stromelysin (MMP-3), and collagenase (MMP-1), continue with the matrix degradation process. Thus, calmodulin antagonists and MMP inhibitors can block resorption by inhibiting acidification of the resorptive compartment [8].
OC are essential for bone destruction. osteoblasts. In inflammatory bone disease, inflammatory cytokines act on osteoblasts and receptor activator of nuclear factor-κB ligand (RANKL)-producing cells, resulting in osteoclast differentiation and activation [9]. Three factors control the cells: M-CSF, RANKL, and osteoprogerine. Apical pole is attached to the ruffle border. It possesses a proton pump ATPasique secreted through a brush border and ionic-exchange channels.
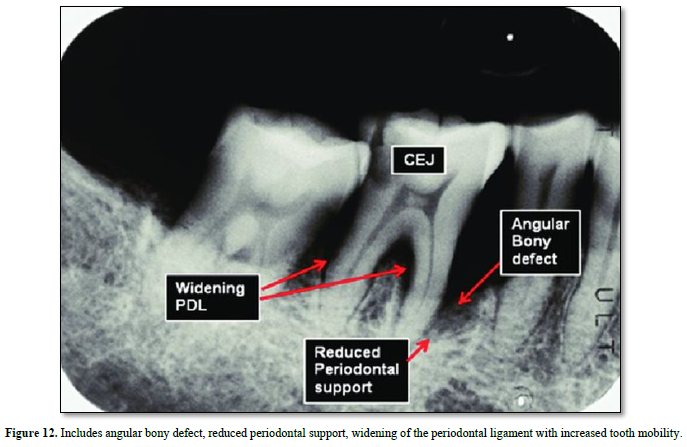
Osteoclast possess both acid excretion exchanges and the apical proton pump V-ATPase Na+/H+ (Figure 12).
Aging
Aging block the translation for cellular senescence promote the degradation of the bone extracellular matrix, and osteoporosis with aging.
Insulin-growth factor-1 (IGF-1) and the growth hormone (GH), whose action is mediated by IGF-1, play a key role in the regulation of the growth, development, metabolic activity and lifespan of several cells and tissues, including bone. Furthermore, IGF-1 plays a key role in osteoclast differentiation. Aging is characterized by a progressive decline in circulating GH and IGF-1 levels, with aging the cellular response to IGF-1 is reduced. Many studies proved that 1.25 (OH)Vitamin D3 stimulation was less effective in increasing the expression of the osteoblastic markers osteocalcin and ALP in osteoblasts derived from aged subjects.
During aging, significant amounts of the bone are lost due to the loss of this delicate balance toward increased bone resorption coupled with decreased formation, which leads to net bone loss of the aging people. Osteoblasts, osteoclasts, and osteocytes are defined by their respective functions of bone formation and bone resorption [10].
Osteoporosis is a systemic bone metabolic disease characterized by destruction of bone microstructure, low bone mineral content, decreased bone strength, and increased brittleness and fracture susceptibility [11].
Commonly used anti-osteoporotic drugs include bisphosphonates, parathyroid hormone, vitamin D, calcium and estrogen. Among them, calcium, vitamin D, simvastatin and parathyroid hormone mainly promote bone formation, and estrogen, bisphosphonates and calcitonin mainly function by inhibiting bone resorption. Some new drugs like simvastatin can improve the high metabolic state of bone, while monoclonal antibody drugs can affect osteoclast activity and have a positive effect on bone reconstruction.
Endocytosis and exocytosis through the ruffled membrane activity contribute to endo/exo cytosis through the membrane limiting the clear zone, migration of vesicles by transcytosis toward the basolateral membrane. Migration of the cells, OC arrest, resorbing phase, and again migration.
CONCLUSIONS
Among the bone diseases linked to OC perturbations, mention should be made to bone osteoporosis, osteogenesis imperfecta, osteopetrosis, and Paget disease. Some of these diseases may be cured by calcium and vitamin D deficiency, bisphosphonates. Treatment of osteopetrosis may be treated by the transplantation of hematopoietic stem cells. Equilibrium between bone formation and destruction are the two dual roles of osteoclasts, the first role being the cell migration out of the Howship lacuna, the other role being OC implication in the turnover of bone extracellular matrix. The release of collagenolytic enzyme and the degradation of the bone extracellular matrix is mainly due to cathepsin K. Additionally, cathepsin B, D, and L are also proteinases that degrade the organic bone matrix, but their effect is not as prominent as cathepsin K. Matrix metalloproteinases (MMPs), especially MMP-9, are also enzymes that degrade the ECM proteins of bone.
No Files Found
Share Your Publication :